Notes Solubility3
Download as ppt, pdf1 like489 views
The document discusses solubility and dissolution rates. It defines solubility as the ability of a substance to dissolve in another, and discusses how "like dissolves like" with polar substances dissolving in polar solvents and nonpolar in nonpolar. It also discusses how to increase dissolution rates through increasing temperature, surface area, and movement/stirring. For gases, it notes they are more soluble at lower temperatures and pressures. It defines unsaturated, saturated, and supersaturated solutions based on concentration levels and discusses how solubility changes with temperature according to solubility charts.
1 of 19
Downloaded 24 times
















Ad
Recommended
Chemistry - Chp 16 - Solutions - PowerPoint (shortened)
Chemistry - Chp 16 - Solutions - PowerPoint (shortened)Mr. Walajtys
?
The document discusses key concepts about solutions, including:
1) The factors that determine whether a substance will dissolve and how much will dissolve include the nature of the solute and solvent, stirring, surface area, and temperature.
2) Solubility is defined as the maximum amount of solute that will dissolve at a specific temperature, and can be expressed in units like grams of solute per 100 grams of solvent.
3) Concentration of a solution can be quantified using molarity, which is defined as moles of solute per liter of solution. More concentrated solutions have a larger amount of solute per amount of solvent.Vocab solutions
Vocab solutionsJeff Kalember
?
The document provides definitions and explanations of key concepts in solutions and solubility including:
- Saturated solutions are holding as much solute as possible, while unsaturated solutions are not holding as much as possible. Dilute solutions have little solute compared to solvent, while concentrated solutions have lots of solute.
- Adding salt raises the boiling point of water. Super saturated solutions hold more solute than normally possible. Water is not the only possible solvent - others include alcohol, antifreeze, mercury and oxygen.
- A solution can be saturated but dilute, like a gold ring in a glass of water. When a liquid dissolves in another liquid, it is called miscible rather thanUnit 10 Solutions Part 2
Unit 10 Solutions Part 2jenneoliver1013
?
The document discusses factors that affect the rate of dissolution of solutes in solvents to form solutions. The three main factors are: surface area, agitation, and temperature. Increasing the surface area of a solute by crushing it provides more contact points for dissolution. Agitation brings fresh solvent into contact with the solute to speed up the process. Temperature also strongly impacts dissolution rates, with higher temperatures causing solutes to dissolve faster due to increased kinetic energy of solvent particles.Unit 10 solutions 1
Unit 10 solutions 1jenneoliver1013
?
A solution is a homogeneous mixture of two or more substances, with a solute that dissolves in a solvent. Water is a universal solvent that can dissolve many other substances like salt or sugar due to its polarity. The concentration of a solution describes how much solute is dissolved in the solvent, and can be saturated, unsaturated, or supersaturated depending on whether it contains the maximum, less than maximum, or more than maximum amount of solute dissolved. Not all solutes are miscible and able to dissolve in a given solvent.Interpreting solubility curves
Interpreting solubility curvesHeidi Cooley
?
This document discusses solubility curves and how they are used to show the solubility of solids and gases in water at different temperatures. Solubility curves indicate the maximum amount of solute that can dissolve in a solvent, with points on the curve showing saturated solutions, above showing supersaturated, and below showing unsaturated. The document explains that for solids, solubility increases with temperature, while for gases solubility decreases with increasing temperature. Examples of solid and gas substances are provided.Changes to sulphur when heated! by axel
Changes to sulphur when heated! by axeljohnwest
?
Sulphur undergoes physical changes as it is heated, starting as a yellow solid and melting into a runny liquid at 113 degrees Celsius, becoming more fluid with continued heating. Upon reaching its boiling point of 445 degrees Celsius, molten sulfur can be poured from its container.ųą╣·┴·ėļėĪČ╚Ž¾
ųą╣·┴·ėļėĪČ╚Ž¾borderless
?
╦µū┼ųą╣·║═ėĪČ╚Ą─┐ņ╦┘ß╚ŲŻ¼╚½Ū“ŠŁ╝├Ė±ŠųĮ½Ęó╔·Žįū┼▒õ╗»Ż¼įż╝ŲĄĮ2050─ĻŻ¼ųą╣·Į½│╔×ķūŅ┤¾Ą─ŠŁ╝├╠ÕŻ¼ėĪČ╚Į¶╦µŲõ║¾ĪŻšŌ┴ĮĖ÷ŠŁ╝├╠ÕĄ─Ęóš╣▓╗Į÷ė░ŽņčuįņęĄ║═Ę■╬±ęĄĄ─Š═ęĄ╗·╗߯¼╗╣┐╔─▄Ą╝ų┬╚½Ū“├│ęū▒Ż╗żų„ęÕĄ─į÷│ż╝░ą┬Ą─└õšĮŠų╩ŲĪŻū„š▀┤¾╬└Īż╩Ę├▄╦╣╠Į╠ų┴╦ųąėĪŠŁ╝├Ą─╠¶šĮėļŪ▒┴”Ż¼▓ó×ķ╣½╦ŠĮ°ę╗▓Į╠Į╦„šŌą®╩ą│Ī╠ß╣®┴╦▒”╣¾Ą─╝¹ĮŌĪŻüĒūįŗīŗī1% Ą─Ė─ūā
üĒūįŗīŗī1% Ą─Ė─ūāborderless
?
▒Š╬─╠Į╠ų┴╦─ĖŪūį┌║óūė│╔╣”ųą╦∙░ńč▌Ą─╣ž╝³ĮŪ╔½Ż¼Ū┐Ą„═©╣²┼Óč°║óūėĄ─╗∙▒Š─▄┴”Ż©╚ńūįųŲ┴”ĪóĮ╗┴„┴”║═š▄č¦╦╝╬¼Ż®└┤┤┘Į°Ųõ│╔│żĪŻū„š▀š┼▒■╗▌┼«╩┐ĘųŽĒ┴╦ČÓųų╩Ąė├╝╝Ū╔║═Į©ęķŻ¼ęį░’ų·ĖĖ─Ėį┌Į╠ė²╣²│╠ųąŻ¼╠ß╔²║óūėĄ─Č└┴óąį║═ė”Čį┤ņš█Ą──▄┴”Ż¼┤ėČ°╬¬║óūėĄ─╚╦╔·ĄņČ©╝ß╩Ą╗∙┤ĪĪŻš¹╠ÕČ°čįŻ¼│╔╣”Ą─╣ž╝³į┌ė┌ĖĖ─ĖĄ─░±č∙┴”┴┐╝░ėąą¦Ą─Į╠ė²ę²Ą╝ĪŻūĘų╚š╣Ō
ūĘų╚š╣Ōborderless
?
ė╚ĮĪż┼Ę┐Ł└¹├µČį═ĒŲ┌─į░®Ą─š’ČŽŻ¼čĪį±Įė╩▄╔·├³Ą─╠¶šĮ▓ó┼¼┴”Į½╩ŻN╩▒╝õ╣²Ą├├└║├ĪŻ╦¹Ę┤╦╝╦└═÷Ą─ęŌęÕŻ¼▓óŽŻ═¹═Ė╣²ūį╝║Ą─ŠŁÜvŻ¼░’ų·╦¹╚╦Ė³║├Ąž└ĒĮŌ╔·├³ėļ╦└═÷ĪŻūŅųšŻ¼╦¹į┌ŲĮŠ▓ųą└ļ╩└Ż¼┴¶Ž┬┴╦ėļŲ▐ūė╣▓═¼ūĘų╣Ō├„Ą─├└║├╗žęõĪŻNotes Solubility2
Notes Solubility2tracy.howard
?
1. A solution is a homogeneous mixture of two or more substances, where the solute is evenly distributed throughout the solvent.
2. The solute is the substance being dissolved and is present in smaller amounts, while the solvent is the dissolving medium and makes up the majority of the solution.
3. Solutions are distinguished from suspensions and colloids by having particles small enough to not be visible, and that can be separated by evaporation but not filtration.├ž├▄
├ž├▄borderless
?
╬³ę²┴”Ę©į“╩ŪėŅųµųąŪ┐┤¾Ą─Ę©į“Ż¼Ū┐Ą„╦╝Žļ║═ŪķĖąĄ─ŲĄ┬╩Čį┤╦Ę©į“Ą─ė░ŽņĪŻ═©╣²╗²╝½╦╝┐╝ĪóĖąČ„║═ūį╬ę░«╗żŻ¼╚╦├Ū┐╔ęį╬³ę²├└║├╩┬╬’Ż¼Ė─▒õ╔·╗ŅĄ─Ė„Ė÷ĘĮ├µĪŻūŅųšŻ¼Ė÷╠Õėą─▄┴”═©╣²ęŌ╩ČčĪį±║═─▄┴┐╣▄└Ē└┤┤┤įņ▓óšŲ┐žūį╝║Ą─├³į╦ĪŻ╚╦╔·č¦ū÷╚╦
╚╦╔·č¦ū÷╚╦borderless
?
╚╦╔·╩Ūę╗│Ī│ųą°Ą─覎░Ż¼ė╚Ųõ╩Ūū÷╚╦ĪŻ╣ž╝³Ą─覎░─┌╚▌░³└©╚Ž┤ĒĪó╚ß║═Īó╔·╚╠Īó╣Ą═©ĪóĘ┼Ž┬║═ĖąČ»Ż¼Ū┐Ą„└ĒĮŌ║═ūųž╦¹╚╦Ż¼ęį┤┘Į°Ė÷╚╦│╔│ż║═╚╦╝╩║═ą│ĪŻūŅųšŻ¼ĮĪ┐ĄĄ─╔·╗ŅĘĮ╩Įę▓╩Ū╔·┤µĄ─ųžę¬ūķ│╔▓┐ĘųĪŻūį╚╗╦╠
ūį╚╗╦╠borderless
?
╬─ĄĄ╠Į╠ų┴╦ūį╚╗ėļ╚╦└Óų«╝õĄ─┴¬ŽĄŻ¼▒Ē┤’┴╦Čįūįė╔ėļĘ╔ŽĶĄ─┐╩═¹ĪŻ═©╣²▒╚ė„Ż¼╚╦└ÓĄ─╣┬Č└║═├╬Žļ▒╗├Ķ╗µ│╔ę╗ųųėļūį╚╗╣▓╬ĶĄ─ūĘŪ¾ĪŻ├┐Ė÷╔·├³Č╝ėąŲõČ└╠žĄ─╩ėĮŪ║═╝█ųĄŻ¼╬▐┬█╔Ē┤”║╬ĄžŻ¼ą─ųąČ╝ėąČį├└║├ėļšµ╩ĄĄ─Ž“═∙ĪŻAlways
Alwaysborderless
?
šŌŲ¬╬─š┬░³║¼╚■Ė÷įóęŌ╔Ņ┐╠Ą─╣╩╩┬Ż¼═©╣²ė──¼Ą─ŪķŠ│š╣╩Š┴╦╬¾┼ą║═ų░│ĪųŪ╗█Ą─ųžę¬ąįĪŻĄ┌ę╗Ė÷╣╩╩┬Ū┐Ą„į┌╬┤┴╦ĮŌšµŽÓŪ░▓╗ė”Ūßęū┼ąČŽŻ¼Ą┌Č■Ė÷╣╩╩┬╠ßąč╚╦├Ūę¬Čįūį╝║Ą─╣żū„▒Ż│ų╩ņŽżŻ¼ęį▒Ń░č╬š╗·╗߯¼Č°Ą┌╚■Ė÷╣╩╩┬į“Į╠Ą╝╚╦├Ūį┌░ņ╣½╩ę│Ī║Žųą┴╦ĮŌ└ŽķøĄ─ųžę¬ąįĪŻšŌą®╣╩╩┬ų╝į┌╠ß╣®╣½╦Š╗ĘŠ│ųąĄ─Į╠čĄ║═Ų¶╩ŠĪŻą▐ąąėą╩«č°
ą▐ąąėą╩«č°borderless
?
ą▐ąąĄ─╩«č°░³║¼č°Ą└Īóč°ąįĪóč°╔±Īóč°ą─Īóč°Ą┬Īóč°ŲĘĪóč°Ų°Īóč°╔ĒĪóč°╔·║═č°Š½Ą╚ĘĮ├µĪŻ├┐ŽŅą▐ąąŪ┐Ą„┴╦─┌į┌Ą─Š│Įńėļą▐č°Ą─ęŌęÕĪŻ═©╣²šŌą®ą▐ąąŻ¼╚╦├Ū┐╔ęį╩ĄŽų╔Ēą─Ą─║═ą│ėļ╔²╗¬ĪŻNotes Solubility1
Notes Solubility1tracy.howard
?
The document discusses the three phases of matter - solids, liquids, and gases. It describes the properties of each phase and how they differ based on particle motion and interaction. In solids, particles are tightly packed and can only vibrate or rotate. Liquids have particles that touch and can slide past one another, taking the shape of their container. Gas particles are independent and do not touch except when colliding. The document also discusses phase changes, intermolecular forces, and how water is an exception as it expands upon freezing.Notes Solubility4
Notes Solubility4tracy.howard
?
This document discusses properties and uses of solutions. It explains that adding a solute affects colligative properties like freezing point and boiling point depression. The freezing point usually decreases and boiling point increases when a solute is added. Concentration is also discussed, with molarity defined as moles of solute per liter of solution. Dilution is described as making a concentrated solution less concentrated. Reverse osmosis is explained as using energy to force water to diffuse against its natural tendency, from an area of lower concentration to higher concentration.Notes Solubility2
Notes Solubility2tracy.howard
?
1. A solution is a homogeneous mixture of two or more substances, where the solute is evenly distributed throughout the solvent. The solute is the substance being dissolved in smaller amounts, while the solvent is the dissolving medium in larger amounts.
2. Solutions have particles too small to be seen, while colloids have intermediate sized particles that can be separated by filters but not boiling. Suspensions have even larger particles that settle out over time.
3. Water is a universal solvent because of its polarity, allowing ionic compounds to dissolve into ions and conduct electricity as electrolytes. Nonpolar compounds generally do not dissolve in water.└┤╚ź╬„▓ž
└┤╚ź╬„▓žborderless
?
šŌŲ¬╬─ĄĄ├Ķ╩÷┴╦ū„š▀į┌2004─Ļ8į┬Ą─╬„▓žų«ąąŻ¼┤ėĘ╔╗·┤░═ŌĄ─ū│╣█Š░╔½ĄĮ╬„▓žĄ─╬─╗»║═╔Ų┴╝Ą─╚╦├±ĪŻū„š▀╠Õčķ┴╦▓žūÕ┤½═│║═ą┼č÷Ż¼Ėą╩▄┴╦╬„▓žĄ─ūį╚╗├└Š░║═╔·╗Ņ╠§╝■Ą─╝Ķ┐ÓĪŻ╬─ųą╠ߥĮĄ─└Ł╚°║═▓╝┤’└Ł╣¼│╔╬¬┴╦┤╦ąąĄ─ėĪŽ¾╔Ņ┐╠Ą─Ž¾š„ĪŻšõ░«╝╣ūĄČÓČÓį╦Č»
šõ░«╝╣ūĄČÓČÓį╦Č»borderless
?
This document provides stretches to do while sitting at a computer to prevent neck, shoulder, and back stiffness. It recommends doing the stretches every hour or whenever feeling stiff. The stretches include neck rolls, shoulder rolls, arm stretches, and leg raises. Doing the stretches regularly while computer working can help the body feel better by countering physical inactivity.Notes Solubility1
Notes Solubility1tracy.howard
?
The document discusses the three phases of matter - solids, liquids, and gases. It describes the properties of each phase and how they differ based on particle motion and interaction. In solids, particles are tightly packed and can only vibrate or rotate. Liquids have particles that can flow past each other but are still in contact. Gas particles are completely independent and rarely touch. It also discusses phase changes between these states of matter and intermolecular forces.Picasso
Picassoborderless
?
▒Ž┐©╦„╩ŪČ■╩«╩└╝═Ą─╬░┤¾ęš╩§╝ęŻ¼╔·ņČ╬„░Óč└Ż¼╩┼╩└ņČĘ©╣·ĪŻ╦¹Ą─╗µ╗ŁĘńĖ±▒õ╗»ČÓČ╦Ż¼ŠŁÜv┴╦└Č╔½╩▒Ų┌ĪóĘ█║ņ╔½╩▒Ų┌ęį╝░┴ó╠Õ┼╔Ą╚▓╗═¼ĮūČ╬Ż¼Ę┤ė│┴╦╦¹Ą─╚╦╔·ŠŁÜv║═ŪķĖąĪŻ╦¹Ą─├¹ū„ĪČĖ±Č¹─ß┐©ĪĘ▒Ē┤’┴╦ČįšĮš∙Ą─┐ž╦▀Ż¼Žį╩Š┴╦╦¹Ą─┤┤įņ┴”ęį╝░Čįęš╩§Ą─╔Ņ┐╠└ĒĮŌĪŻ7Ė÷ąĪŽĖĮ┌╗┘Ą¶─Ń╔Ē╠Õ
7Ė÷ąĪŽĖĮ┌╗┘Ą¶─Ń╔Ē╠Õborderless
?
╬─ĄĄ┴ą│÷┴╦Ų▀Ė÷▓╗┴╝╔·╗ŅŽ░╣▀Ż¼šŌą®Ž░╣▀┐╔─▄Čį╔Ē╠ÕĮĪ┐Ąįņ│╔čŽųžė░ŽņĪŻ░³└©╝óČ÷Īó┐┌┐╩ĪóŲŻŠļĄ╚Ūķ┐÷║¾▓┼▓╔╚ĪąąČ»Ą─ū÷Ę©Ż¼┐╔─▄Ą╝ų┬Ž¹╗»Ą└║═├Ōę▀ŽĄ═│Ą─╦║”ĪŻ╬─╝■Ū┐Ą„Č©╩▒ę¹╩│Īóę¹╦«Īóą▌Žó║═ū„ŽóĄ─ųžę¬ąįŻ¼ęįįżĘ└╝▓▓ĪĄ─Ęó╔·ĪŻNotes Solubility3
Notes Solubility3tracy.howard
?
The document discusses solubility and dissolution rates. It defines solubility as the ability of a substance to dissolve in another, and discusses how "like dissolves like" with polar substances dissolving in polar solvents and nonpolar in nonpolar. It also discusses how to increase dissolution rates, such as by increasing temperature, surface area, or movement. For gases, dissolution is increased at lower temperatures and higher pressures. Concentration of solutions is also covered, defining unsaturated, saturated, and supersaturated solutions based on how close they are to the maximum solubility for a given temperature.7 Science Chapter 8.ppt
7 Science Chapter 8.pptTcherReaQuezada
?
This document provides an overview of solutions and factors that affect the dissolving process. It defines a solution as a homogeneous mixture that appears as a single substance. Solutions are made up of a solute, the substance that dissolves, and a solvent, the substance the solute dissolves in. The rate at which a solute dissolves, or its solubility, depends on properties like temperature, pressure, the size of solute particles, and stirring. Higher temperatures typically increase solubility for solids but decrease it for gases. Smaller solute particles and higher pressures also increase solubility.Properties of Solutions
Properties of SolutionsMelinda MacDonald
?
This document discusses key properties and concepts related to solutions. It defines a solution as being made up of a solvent and one or more solutes. The solvent is the substance that exists in the greatest quantity, while solutes are all other substances present. Solutions can be solid, liquid or gas depending on the state of the solvent. The document also discusses concentration, solubility, factors that affect solubility like temperature and pressure, and how to increase the rate at which a solute dissolves.Dacota_blue K12: Science 7: Quarter 1: Module 1 matter
Dacota_blue K12: Science 7: Quarter 1: Module 1 matterDaniel Tabinga
?
This document discusses solutions and related concepts. It begins by defining matter and the different states of matter. It then discusses solutions in depth, including the components of solutions, different types of solutions, factors that affect solubility rates, and ways to express concentration. Examples are provided throughout to illustrate these concepts. Concentration is discussed further, including percentage by weight and by volume. Factors that affect how quickly solutes dissolve are also outlined.Mixtures
Mixturescharsh
?
The document discusses different types of mixtures including solutions, suspensions, and colloids. It explains that a solution is a homogeneous mixture of two or more substances where a solute dissolves evenly throughout a solvent. Suspensions are heterogeneous mixtures where insoluble particles settle out of the mixture. Colloids have intermediate particle sizes that do not settle out.SCIENCE-9-APPLIED-CHEMISTRY-UNIT-4.1.1-TYPES-OF-SOLUTIONS-NOTES.pptx
SCIENCE-9-APPLIED-CHEMISTRY-UNIT-4.1.1-TYPES-OF-SOLUTIONS-NOTES.pptxysla owo
?
The document provides an overview of solutions, defining them as homogeneous mixtures of solutes and solvents and categorizing them based on solubility, concentration, and physical state. Key characteristics include uniform composition, stability, and the absence of the Tyndall effect. It also discusses various examples of solutions encountered in daily life, such as saltwater and syrupy beverages, as well as the factors influencing solubility.Solution.pdf
Solution.pdfAbegailDimaano8
?
This document defines and compares saturated, unsaturated, and supersaturated solutions. It explains that a saturated solution contains the maximum amount of solute that can dissolve in a given amount of solvent at a certain temperature. An unsaturated solution contains less solute than this maximum. A supersaturated solution contains more solute than the maximum but the solute remains dissolved due to elevated temperature; cooling causes precipitation until saturation is reached. The document also examines how temperature affects solubility through a kitchen experiment comparing hot and cold water solutions.More Related Content
Viewers also liked (16)
ūĘų╚š╣Ō
ūĘų╚š╣Ōborderless
?
ė╚ĮĪż┼Ę┐Ł└¹├µČį═ĒŲ┌─į░®Ą─š’ČŽŻ¼čĪį±Įė╩▄╔·├³Ą─╠¶šĮ▓ó┼¼┴”Į½╩ŻN╩▒╝õ╣²Ą├├└║├ĪŻ╦¹Ę┤╦╝╦└═÷Ą─ęŌęÕŻ¼▓óŽŻ═¹═Ė╣²ūį╝║Ą─ŠŁÜvŻ¼░’ų·╦¹╚╦Ė³║├Ąž└ĒĮŌ╔·├³ėļ╦└═÷ĪŻūŅųšŻ¼╦¹į┌ŲĮŠ▓ųą└ļ╩└Ż¼┴¶Ž┬┴╦ėļŲ▐ūė╣▓═¼ūĘų╣Ō├„Ą─├└║├╗žęõĪŻNotes Solubility2
Notes Solubility2tracy.howard
?
1. A solution is a homogeneous mixture of two or more substances, where the solute is evenly distributed throughout the solvent.
2. The solute is the substance being dissolved and is present in smaller amounts, while the solvent is the dissolving medium and makes up the majority of the solution.
3. Solutions are distinguished from suspensions and colloids by having particles small enough to not be visible, and that can be separated by evaporation but not filtration.├ž├▄
├ž├▄borderless
?
╬³ę²┴”Ę©į“╩ŪėŅųµųąŪ┐┤¾Ą─Ę©į“Ż¼Ū┐Ą„╦╝Žļ║═ŪķĖąĄ─ŲĄ┬╩Čį┤╦Ę©į“Ą─ė░ŽņĪŻ═©╣²╗²╝½╦╝┐╝ĪóĖąČ„║═ūį╬ę░«╗żŻ¼╚╦├Ū┐╔ęį╬³ę²├└║├╩┬╬’Ż¼Ė─▒õ╔·╗ŅĄ─Ė„Ė÷ĘĮ├µĪŻūŅųšŻ¼Ė÷╠Õėą─▄┴”═©╣²ęŌ╩ČčĪį±║═─▄┴┐╣▄└Ē└┤┤┤įņ▓óšŲ┐žūį╝║Ą─├³į╦ĪŻ╚╦╔·č¦ū÷╚╦
╚╦╔·č¦ū÷╚╦borderless
?
╚╦╔·╩Ūę╗│Ī│ųą°Ą─覎░Ż¼ė╚Ųõ╩Ūū÷╚╦ĪŻ╣ž╝³Ą─覎░─┌╚▌░³└©╚Ž┤ĒĪó╚ß║═Īó╔·╚╠Īó╣Ą═©ĪóĘ┼Ž┬║═ĖąČ»Ż¼Ū┐Ą„└ĒĮŌ║═ūųž╦¹╚╦Ż¼ęį┤┘Į°Ė÷╚╦│╔│ż║═╚╦╝╩║═ą│ĪŻūŅųšŻ¼ĮĪ┐ĄĄ─╔·╗ŅĘĮ╩Įę▓╩Ū╔·┤µĄ─ųžę¬ūķ│╔▓┐ĘųĪŻūį╚╗╦╠
ūį╚╗╦╠borderless
?
╬─ĄĄ╠Į╠ų┴╦ūį╚╗ėļ╚╦└Óų«╝õĄ─┴¬ŽĄŻ¼▒Ē┤’┴╦Čįūįė╔ėļĘ╔ŽĶĄ─┐╩═¹ĪŻ═©╣²▒╚ė„Ż¼╚╦└ÓĄ─╣┬Č└║═├╬Žļ▒╗├Ķ╗µ│╔ę╗ųųėļūį╚╗╣▓╬ĶĄ─ūĘŪ¾ĪŻ├┐Ė÷╔·├³Č╝ėąŲõČ└╠žĄ─╩ėĮŪ║═╝█ųĄŻ¼╬▐┬█╔Ē┤”║╬ĄžŻ¼ą─ųąČ╝ėąČį├└║├ėļšµ╩ĄĄ─Ž“═∙ĪŻAlways
Alwaysborderless
?
šŌŲ¬╬─š┬░³║¼╚■Ė÷įóęŌ╔Ņ┐╠Ą─╣╩╩┬Ż¼═©╣²ė──¼Ą─ŪķŠ│š╣╩Š┴╦╬¾┼ą║═ų░│ĪųŪ╗█Ą─ųžę¬ąįĪŻĄ┌ę╗Ė÷╣╩╩┬Ū┐Ą„į┌╬┤┴╦ĮŌšµŽÓŪ░▓╗ė”Ūßęū┼ąČŽŻ¼Ą┌Č■Ė÷╣╩╩┬╠ßąč╚╦├Ūę¬Čįūį╝║Ą─╣żū„▒Ż│ų╩ņŽżŻ¼ęį▒Ń░č╬š╗·╗߯¼Č°Ą┌╚■Ė÷╣╩╩┬į“Į╠Ą╝╚╦├Ūį┌░ņ╣½╩ę│Ī║Žųą┴╦ĮŌ└ŽķøĄ─ųžę¬ąįĪŻšŌą®╣╩╩┬ų╝į┌╠ß╣®╣½╦Š╗ĘŠ│ųąĄ─Į╠čĄ║═Ų¶╩ŠĪŻą▐ąąėą╩«č°
ą▐ąąėą╩«č°borderless
?
ą▐ąąĄ─╩«č°░³║¼č°Ą└Īóč°ąįĪóč°╔±Īóč°ą─Īóč°Ą┬Īóč°ŲĘĪóč°Ų°Īóč°╔ĒĪóč°╔·║═č°Š½Ą╚ĘĮ├µĪŻ├┐ŽŅą▐ąąŪ┐Ą„┴╦─┌į┌Ą─Š│Įńėļą▐č°Ą─ęŌęÕĪŻ═©╣²šŌą®ą▐ąąŻ¼╚╦├Ū┐╔ęį╩ĄŽų╔Ēą─Ą─║═ą│ėļ╔²╗¬ĪŻNotes Solubility1
Notes Solubility1tracy.howard
?
The document discusses the three phases of matter - solids, liquids, and gases. It describes the properties of each phase and how they differ based on particle motion and interaction. In solids, particles are tightly packed and can only vibrate or rotate. Liquids have particles that touch and can slide past one another, taking the shape of their container. Gas particles are independent and do not touch except when colliding. The document also discusses phase changes, intermolecular forces, and how water is an exception as it expands upon freezing.Notes Solubility4
Notes Solubility4tracy.howard
?
This document discusses properties and uses of solutions. It explains that adding a solute affects colligative properties like freezing point and boiling point depression. The freezing point usually decreases and boiling point increases when a solute is added. Concentration is also discussed, with molarity defined as moles of solute per liter of solution. Dilution is described as making a concentrated solution less concentrated. Reverse osmosis is explained as using energy to force water to diffuse against its natural tendency, from an area of lower concentration to higher concentration.Notes Solubility2
Notes Solubility2tracy.howard
?
1. A solution is a homogeneous mixture of two or more substances, where the solute is evenly distributed throughout the solvent. The solute is the substance being dissolved in smaller amounts, while the solvent is the dissolving medium in larger amounts.
2. Solutions have particles too small to be seen, while colloids have intermediate sized particles that can be separated by filters but not boiling. Suspensions have even larger particles that settle out over time.
3. Water is a universal solvent because of its polarity, allowing ionic compounds to dissolve into ions and conduct electricity as electrolytes. Nonpolar compounds generally do not dissolve in water.└┤╚ź╬„▓ž
└┤╚ź╬„▓žborderless
?
šŌŲ¬╬─ĄĄ├Ķ╩÷┴╦ū„š▀į┌2004─Ļ8į┬Ą─╬„▓žų«ąąŻ¼┤ėĘ╔╗·┤░═ŌĄ─ū│╣█Š░╔½ĄĮ╬„▓žĄ─╬─╗»║═╔Ų┴╝Ą─╚╦├±ĪŻū„š▀╠Õčķ┴╦▓žūÕ┤½═│║═ą┼č÷Ż¼Ėą╩▄┴╦╬„▓žĄ─ūį╚╗├└Š░║═╔·╗Ņ╠§╝■Ą─╝Ķ┐ÓĪŻ╬─ųą╠ߥĮĄ─└Ł╚°║═▓╝┤’└Ł╣¼│╔╬¬┴╦┤╦ąąĄ─ėĪŽ¾╔Ņ┐╠Ą─Ž¾š„ĪŻšõ░«╝╣ūĄČÓČÓį╦Č»
šõ░«╝╣ūĄČÓČÓį╦Č»borderless
?
This document provides stretches to do while sitting at a computer to prevent neck, shoulder, and back stiffness. It recommends doing the stretches every hour or whenever feeling stiff. The stretches include neck rolls, shoulder rolls, arm stretches, and leg raises. Doing the stretches regularly while computer working can help the body feel better by countering physical inactivity.Notes Solubility1
Notes Solubility1tracy.howard
?
The document discusses the three phases of matter - solids, liquids, and gases. It describes the properties of each phase and how they differ based on particle motion and interaction. In solids, particles are tightly packed and can only vibrate or rotate. Liquids have particles that can flow past each other but are still in contact. Gas particles are completely independent and rarely touch. It also discusses phase changes between these states of matter and intermolecular forces.Picasso
Picassoborderless
?
▒Ž┐©╦„╩ŪČ■╩«╩└╝═Ą─╬░┤¾ęš╩§╝ęŻ¼╔·ņČ╬„░Óč└Ż¼╩┼╩└ņČĘ©╣·ĪŻ╦¹Ą─╗µ╗ŁĘńĖ±▒õ╗»ČÓČ╦Ż¼ŠŁÜv┴╦└Č╔½╩▒Ų┌ĪóĘ█║ņ╔½╩▒Ų┌ęį╝░┴ó╠Õ┼╔Ą╚▓╗═¼ĮūČ╬Ż¼Ę┤ė│┴╦╦¹Ą─╚╦╔·ŠŁÜv║═ŪķĖąĪŻ╦¹Ą─├¹ū„ĪČĖ±Č¹─ß┐©ĪĘ▒Ē┤’┴╦ČįšĮš∙Ą─┐ž╦▀Ż¼Žį╩Š┴╦╦¹Ą─┤┤įņ┴”ęį╝░Čįęš╩§Ą─╔Ņ┐╠└ĒĮŌĪŻ7Ė÷ąĪŽĖĮ┌╗┘Ą¶─Ń╔Ē╠Õ
7Ė÷ąĪŽĖĮ┌╗┘Ą¶─Ń╔Ē╠Õborderless
?
╬─ĄĄ┴ą│÷┴╦Ų▀Ė÷▓╗┴╝╔·╗ŅŽ░╣▀Ż¼šŌą®Ž░╣▀┐╔─▄Čį╔Ē╠ÕĮĪ┐Ąįņ│╔čŽųžė░ŽņĪŻ░³└©╝óČ÷Īó┐┌┐╩ĪóŲŻŠļĄ╚Ūķ┐÷║¾▓┼▓╔╚ĪąąČ»Ą─ū÷Ę©Ż¼┐╔─▄Ą╝ų┬Ž¹╗»Ą└║═├Ōę▀ŽĄ═│Ą─╦║”ĪŻ╬─╝■Ū┐Ą„Č©╩▒ę¹╩│Īóę¹╦«Īóą▌Žó║═ū„ŽóĄ─ųžę¬ąįŻ¼ęįįżĘ└╝▓▓ĪĄ─Ęó╔·ĪŻNotes Solubility3
Notes Solubility3tracy.howard
?
The document discusses solubility and dissolution rates. It defines solubility as the ability of a substance to dissolve in another, and discusses how "like dissolves like" with polar substances dissolving in polar solvents and nonpolar in nonpolar. It also discusses how to increase dissolution rates, such as by increasing temperature, surface area, or movement. For gases, dissolution is increased at lower temperatures and higher pressures. Concentration of solutions is also covered, defining unsaturated, saturated, and supersaturated solutions based on how close they are to the maximum solubility for a given temperature.Similar to Notes Solubility3 (20)
7 Science Chapter 8.ppt
7 Science Chapter 8.pptTcherReaQuezada
?
This document provides an overview of solutions and factors that affect the dissolving process. It defines a solution as a homogeneous mixture that appears as a single substance. Solutions are made up of a solute, the substance that dissolves, and a solvent, the substance the solute dissolves in. The rate at which a solute dissolves, or its solubility, depends on properties like temperature, pressure, the size of solute particles, and stirring. Higher temperatures typically increase solubility for solids but decrease it for gases. Smaller solute particles and higher pressures also increase solubility.Properties of Solutions
Properties of SolutionsMelinda MacDonald
?
This document discusses key properties and concepts related to solutions. It defines a solution as being made up of a solvent and one or more solutes. The solvent is the substance that exists in the greatest quantity, while solutes are all other substances present. Solutions can be solid, liquid or gas depending on the state of the solvent. The document also discusses concentration, solubility, factors that affect solubility like temperature and pressure, and how to increase the rate at which a solute dissolves.Dacota_blue K12: Science 7: Quarter 1: Module 1 matter
Dacota_blue K12: Science 7: Quarter 1: Module 1 matterDaniel Tabinga
?
This document discusses solutions and related concepts. It begins by defining matter and the different states of matter. It then discusses solutions in depth, including the components of solutions, different types of solutions, factors that affect solubility rates, and ways to express concentration. Examples are provided throughout to illustrate these concepts. Concentration is discussed further, including percentage by weight and by volume. Factors that affect how quickly solutes dissolve are also outlined.Mixtures
Mixturescharsh
?
The document discusses different types of mixtures including solutions, suspensions, and colloids. It explains that a solution is a homogeneous mixture of two or more substances where a solute dissolves evenly throughout a solvent. Suspensions are heterogeneous mixtures where insoluble particles settle out of the mixture. Colloids have intermediate particle sizes that do not settle out.SCIENCE-9-APPLIED-CHEMISTRY-UNIT-4.1.1-TYPES-OF-SOLUTIONS-NOTES.pptx
SCIENCE-9-APPLIED-CHEMISTRY-UNIT-4.1.1-TYPES-OF-SOLUTIONS-NOTES.pptxysla owo
?
The document provides an overview of solutions, defining them as homogeneous mixtures of solutes and solvents and categorizing them based on solubility, concentration, and physical state. Key characteristics include uniform composition, stability, and the absence of the Tyndall effect. It also discusses various examples of solutions encountered in daily life, such as saltwater and syrupy beverages, as well as the factors influencing solubility.Solution.pdf
Solution.pdfAbegailDimaano8
?
This document defines and compares saturated, unsaturated, and supersaturated solutions. It explains that a saturated solution contains the maximum amount of solute that can dissolve in a given amount of solvent at a certain temperature. An unsaturated solution contains less solute than this maximum. A supersaturated solution contains more solute than the maximum but the solute remains dissolved due to elevated temperature; cooling causes precipitation until saturation is reached. The document also examines how temperature affects solubility through a kitchen experiment comparing hot and cold water solutions.Elements Compounds And Mixtures
Elements Compounds And Mixturesdeawscience
?
This document provides an overview of acids, bases, and solutions. It defines acids as substances that release hydrogen ions in water, causing solutions to have a sour taste and be able to conduct electricity. Bases are defined as substances that accept hydrogen ions in water, releasing hydroxide ions and causing solutions to feel slippery and taste bitter. The document also discusses pH scale, acid and base strengths, indicators, and neutralization reactions between acids and bases.Solutions
SolutionsCurrituck County High School
?
This document defines key concepts related to solutions, including:
- A solution is a homogeneous mixture of a solvent and one or more solutes. Common solvents are water.
- The process of dissolving a solute involves the solute and solvent particles separating and the solvent surrounding and solvating the solute particles. This process can be endothermic or exothermic.
- Solubility depends on properties of the solute and solvent as well as temperature and pressure. Solubility curves show how solubility changes with temperature. Saturation occurs when a solution contains the maximum amount of solute possible.Online Solutions
Online SolutionsHwa Chong Institution
?
This document provides information about solutions and solubility. It defines key terms like solute, solvent, saturated solution, and solubility. It explains that a solution is a homogeneous mixture of substances where one substance dissolves in another. Water is called the universal solvent because it can dissolve more substances than any other solvent. Not all substances are soluble and solubility depends on temperature, the nature of the solute and solvent, and other factors. The document also discusses different types of solutions and rates of dissolving.Solution
SolutionJazmin April Pescasio
?
This document defines key terms related to solutions and solubility. It discusses the components of solutions, different types of solutions based on the state of the solvent, and factors that affect solubility such as temperature, pressure, and the nature of the solute and solvent. Solubility is the maximum amount of solute that can dissolve in a solvent under given conditions.Solution
SolutionJazmin April Pescasio
?
This document defines key terms related to solutions and solubility. It discusses the components of solutions, different types of solutions based on the state of the solvent, and factors that affect the dissolving process and solubility of solutes, including temperature, pressure, and the nature of the solute and solvent. It also defines terms like unsaturated, saturated, and supersaturated to describe how much solute a solution can hold.Chem 1 unit 11 presentation
Chem 1 unit 11 presentationbobcatchemistry
?
Solutions can be either homogeneous or heterogeneous mixtures. Concentration of solutions can be expressed in terms of percent, moles, molarity, or molality. Factors such as temperature, pressure, and polarity affect the solubility and formation of solutions. Colligative properties depend on the number of solute particles in solution. Stoichiometry can be used to solve solution reaction problems by considering the concentrations of reactants and products.Chem 1 unit 11 presentation
Chem 1 unit 11 presentationbobcatchemistry
?
Solutions can be either homogeneous, made of one phase, or heterogeneous, made of two or more distinct phases. Concentration of a solution can be expressed in terms of percent composition, molarity, or molality. Factors such as temperature, pressure, and polarity affect the solubility and formation of solutions. Stoichiometry can be used to solve problems involving the concentrations of reactants and products in solution.Chemistry project for Class 12 boards
Chemistry project for Class 12 boardsNIKHIL DUGGAL
?
This document describes a student project measuring the solubility of sodium chloride, magnesium sulfate, and sucrose in water. The student followed an experimental procedure that involved adding incremental amounts of each solute to water until saturation was reached. Precautions were taken to ensure consistent temperature and stirring. The results found that sodium chloride had the highest solubility due to its small, dissociated ions, while sucrose had the lowest solubility due to its large molecular structure. Adding heat increased the solubility of all solutes by providing more kinetic energy to molecules. The conclusions and results aligned with chemical theories of solubility.Chemistryproject 170204054007
Chemistryproject 170204054007NISHANTSINGH411
?
This document describes a student project measuring the solubility of common chemicals like table salt, Epsom salts, and sugar in water. The student followed an experimental procedure that involved adding incremental amounts of each chemical to water until saturation was reached. Key results were that table salt had the highest solubility due to its small ionic components, while sugar had the lowest solubility due to its large molecular size. Increasing the temperature increased the solubility of all chemicals by providing more kinetic energy to molecules. The student's results and conclusions agreed with chemical theories of solubility.Concentration2008
Concentration2008tams
?
The document compares saturated, unsaturated, and supersaturated solutions. It defines concentration and discusses calculating concentration by mass and volume. It also discusses factors that increase the rate of dissolving, such as heating and stirring, and provides examples of different types of solutions.Chem 1 unit 11 presentation
Chem 1 unit 11 presentationbobcatchemistry
?
Solutions can be either homogeneous, made of one phase, or heterogeneous, made of two or more distinct phases. Concentration of a solution can be expressed in terms of percent composition, molarity, or molality. Factors such as temperature, pressure, and polarity affect the solubility and formation of solutions. Stoichiometry can be used to solve problems involving reactions in solution by using molar concentrations.Chem 1 unit 11 presentation
Chem 1 unit 11 presentationbobcatchemistry
?
* Given: 0.85 g of gas dissolves in 1 L of water at 4 atm
* Let's call the amount that will dissolve at 1 atm = S1
* Using Henry's Law: S1/S2 = P1/P2
* S2 is the amount we want to find at 1 atm
* P1 = 4 atm
* P2 = 1 atm
* S1 = 0.85 g
* Substitute into Henry's Law equation:
* 0.85/S2 = 4/1
* S2 = 0.85 * 1/4 = 0.2125 g
Therefore, the amount that will dissolve in 1 L of water at 1 atm isChemunit11presentation 120308075246-phpapp01
Chemunit11presentation 120308075246-phpapp01Cleophas Rwemera
?
This document discusses solutions and factors that affect their formation and properties. It defines key terms like heterogeneous and homogeneous mixtures, concentration, solubility, and Henry's Law. Solution stoichiometry problems can be solved using concentration equations to determine amounts of reactants and products. Factors like temperature, pressure, and intermolecular forces influence a substance's solubility in a given solvent.Unit 15 Power Point
Unit 15 Power PointOlympus High School - Jeff Taylor
?
1. A solution is a mixture of two or more substances, where the substance that dissolves the others is called the solvent and the substances that dissolve are called solutes. For example, in salt water the solvent is water and the solute is salt.
2. Solubility is affected by temperature - more solute can dissolve in hot solvents compared to cool solvents. At saturation, cooling a solution may cause crystals to precipitate out.
3. Electrolytes, such as salts, dissociate into ions when dissolved in water and allow the solution to conduct electricity. Nonelectrolytes like sugar do not dissociate or conduct.
4. Adding a soluteAd
Recently uploaded (20)
Marketing Assignment presentation of good marketing techniques how to impleme...
Marketing Assignment presentation of good marketing techniques how to impleme...Priya Raj
?
Marketing presentationWebinar: Why Odoo is a game-changer for Service Companies
Webinar: Why Odoo is a game-changer for Service Companiesdear digital
?
Watch the webinar: https://youtu.be/49xUiOHJwa4
Running a service business? Then you know how messy operations can get.
YouĪ»re not selling products. YouĪ»re selling time, expertise, and client satisfaction. That means your tools need to handle projects, people, planning, and billing - all in one place.
ThatĪ»s exactly where Odoo shines. Unlike traditional ERP systems, Odoo is built to support the unique workflows of service companies. From managing projects and tracking time to invoicing, signing contracts, and handling support tickets: Odoo brings everything together. No more jumping between a plethora of spreadsheets, tools, and inboxes.
With over 45 modules, Odoo grows with your business. Need CRM today, Helpdesk tomorrow? No worries, itĪ»s all connected. Plus, itĪ»s fully customizable to match the way your company works.
Wondering if itĪ»s a fit?
Join our 1-hour webinar where our Odoo expert Julien will walk you through the power of Odoo for service companies, complete with real-life examples from companies like yours!
What youĪ»ll learn:
? How Odoo streamlines the entire service lifecycle
? Key modules for service businesses: Project, Timesheets, CRM, Invoicing & more
? How reporting works within Odoo
? What makes Odoo different from other ERP systems
? When Odoo is a fit for your company (and when it's not)
? A demo where we guide you through the possibilities step-by-step
Stuart Frost - The Chief Executive Officer Of Geminos
Stuart Frost - The Chief Executive Officer Of GeminosStuart Frost
?
Stuart Frost, CEO of Geminos, is a pioneering force in the IIoT and data analytics industry. After graduating from Nottingham University in Electronic and Computer Engineering, Stu founded SELECT Software Tools and led it through a NASDAQ IPO in 1996. His ventures in the IIoT space include Maana, OspreyData, and SWARM. IT Companies in Magarpatta: A Thriving Hub of Technology and Innovation
IT Companies in Magarpatta: A Thriving Hub of Technology and Innovationprernarathi90
?
IT Firms in Magarpatta have transformed the region into one of PuneĪ»s most vibrant and sought-after technology destinations. Located in Hadapsar, Magarpatta City is a privately managed, self-sustained township that blends residential, commercial, and IT infrastructure with green and eco-friendly planning. The presence of leading software companies, excellent connectivity, and world-class amenities make it a hotspot for IT professionals and businesses alike.
Enhancing Customer Engagement in Direct Selling Through AI Segmentation
Enhancing Customer Engagement in Direct Selling Through AI SegmentationEpixel MLM Software
?
Direct selling is a successful business model where they always incorporate and upgrade themselves with latest strategies and trends. Customers preferences and needs always change. So, it is essential for the companies to understand the changes from the beginning itself and provide them the personalized services.
Employing AI segmentation in direct selling ensures the companies increased customer satisfaction and customer engagement. With AI, companies can get their customers individual preferences, interactions, and even emotional cues. By integrating AI with direct sales, companies can benefit such as analyzing individual behaviors, preferences, and emotional cues, providing deeper insights of each customer, enabling personalized engagement with the audience, anticipating future customer behavior based on historical data, and providing more precise customer targeting by identifying pattern.
Direct selling is all about micro moments small, often fleeting, opportunities to engage with customers. Companies using AI can pinpoint these moments and understand customer preferences on their purchase patterns and provide them the response in the most relevant and timely way. Paul Turovsky - A Key Contributor
Paul Turovsky - A Key ContributorPaul Turovsky
?
Paul Turovsky is a manager for Foursquare Realty Investments. He graduated from Ave Maria School of Law in 2013 and received his undergraduate degree from Baruch College. Mr. Turovsky has founded multiple real estate firms to serve various functions. Ihor Pavlenko: ¦Ą¦ß¦Ō¦č¦ė¦▌?¦▀¦▀¦± ¦Ō¦┌¦┘¦┌¦▄¦č¦▐¦┌ ¦┘¦č ¦š¦Ó¦ß¦Ó¦▐¦Ó¦į¦Ó¦ AI (UA)
Ihor Pavlenko: ¦Ą¦ß¦Ō¦č¦ė¦▌?¦▀¦▀¦± ¦Ō¦┌¦┘¦┌¦▄¦č¦▐¦┌ ¦┘¦č ¦š¦Ó¦ß¦Ó¦▐¦Ó¦į¦Ó¦ AI (UA)Lviv Startup Club
?
Ihor Pavlenko: ¦Ą¦ß¦Ō¦č¦ė¦▌?¦▀¦▀¦± ¦Ō¦┌¦┘¦┌¦▄¦č¦▐¦┌ ¦┘¦č ¦š¦Ó¦ß¦Ó¦▐¦Ó¦į¦Ó¦ AI (UA)
LemBS AI PM School 2025
Website ©C https://lembs.com/aipmschool
Youtube ©C https://www.youtube.com/startuplviv
FB ©C https://www.facebook.com/pmdayconferenceBook - Behavioral finance and wealth management(1).pdf
Book - Behavioral finance and wealth management(1).pdfGamingwithUBAID
?
Book - Behavioral finance and wealth management(1).pdfStone Hill Ready Mix Concrete Bagalur
Stone Hill Ready Mix Concrete Bagalurstonehillrealtyblr
?
Stone Hill Ready Mix Concrete is a trusted provider of high-quality ready-mix concrete solutions, serving construction and infrastructure projects across Bagalur. With a commitment to precision, durability, and customer satisfaction, we specialize in supplying concrete tailored to meet the unique requirements of residential, commercial, and industrial projects.
Pendant Lights That Reflect Your Style and Transform Your Living Space
Pendant Lights That Reflect Your Style and Transform Your Living Spaceneslightingofficial
?
Discover how pendant lighting from NES Lighting can transform your living space. This 5-slide presentation highlights the elegance, practicality, and versatility of pendant lightsĪ¬from stylish space-saving designs to luxurious crystal fixtures. Whether you're renovating a cozy room or modernizing your entire home, NES Lighting offers smart, easy-to-install solutions to match any decor. Perfect for customers, partners, or design enthusiasts looking to elevate interiors with lighting that makes a statement.Ak?n©¬ pl©ón pro chemick? pr?mysl - Ivan Sou?ek
Ak?n©¬ pl©ón pro chemick? pr?mysl - Ivan Sou?ekpavelborek
?
Kulat? st?l: CLEAN INDUSTRIAL DEAL ©C BUDOUCNOST ?ESK? CHEMIE A OCEL??STV? se uskute?nil 18.6.2025 v s©¬dle HK ?RERP Modernization in 2025: A Practical Guide for CIOs
ERP Modernization in 2025: A Practical Guide for CIOsPraxis Info Solutions
?
Many companies still use old, on-premises ERP systems.
This creates technical debt and higher maintenance costs.
ERP Modernization helps move systems to the cloud, improving flexibility and access.smidmart industrial Automation Ones Stop Solution
smidmart industrial Automation Ones Stop Solutionsmidmart
?
Smidmart is an industrial automation products one stop solutions where you got all materials you want from control panel cutomization to checkweigher parts your one stop solution is smidart . we are avialble on https://www.smidmart.com/Miriam Cho: Transforming Healthcare through Visionary Leadership
Miriam Cho: Transforming Healthcare through Visionary Leadershipjessicashaw101998
?
Miriam Cho, President and Chief Pharmacy Officer (CPO) of MAC Rx, LLC, has extensive experience in the LTC and healthcare sectors. Miriam is skilled in various areas, including healthcare management, pharmacy operations, cost management, Medicare Part D, cardiopulmonary resuscitation (CPR), pharmacy consulting, and clinical pharmacology. She holds a Doctor of Pharmacy (Pharm.D.) degree from Midwestern University in Illinois, showcasing her strong background in healthcare services.StarOps- AI-native platform that lets you deploy and manage production infras...
StarOps- AI-native platform that lets you deploy and manage production infras...StarOps
?
StarOps is an AI-powered workflow engine that lets you deploy, manage, and scale your application infrastructure - without writing a single Terraform file or managing Kubernetes manually.
Whether you're launching a GenAI model, provisioning blob storage, configuring VPCs, or setting up observability, StarOps handles the cloud operational complexity for you. ItĪ»s like having a team of microagents managing your infrastructure behind the scenes, purpose-built for the new wave of AI and data-heavy applications.
Who ItĪ»s For:
- Application developers who want infrastructure that just works.
- ML engineers and data scientists who want to ship models without devops blockers.
- Platform engineers who want to scale their teams, not their workload.
Start your Free Trial here: https://ingenimax.ai/Zero-emission zones in the Netherlands 2025
Zero-emission zones in the Netherlands 2025Walther Ploos van Amstel
?
Since 2014, the Netherlands has worked toward more sustainable urban logistics through the Green Deal Zero Emission City Logistics. Early years focused on local pilot projectsĪ¬such as city hubs, electric vehicles, and water transportĪ¬designed as Ī▒living labsĪ▒ to explore and learn. Following the 2019 Climate Agreement, the ambition increased: by 2025, 30 to 40 major municipalities aim to establish zero-emission zones, where only fully electric trucks and vans may operate. As of January 2025, 14 cities have implemented such zones, banning vehicles with Euro 4 or lower emission standards. By 2030, only zero-emission vehicles will be permitted.
The rollout raises important questions: Which transitions are smooth? Where are the main challenges, and who is most affected? Are issues local or widespread? How are small and large companies affected differently? Researchers play a vital role in evaluating these developments, sharing insights, and supporting a just and effective transition to zero-emission city logistics.
Walther Ploos van Amstel.Electronic Shelf Labels & Digital Price Tags in Delhi | Foodcare
Electronic Shelf Labels & Digital Price Tags in Delhi | FoodcareFoodcare llp
?
Foodcare brings advanced electronic shelf labels and digital price tags to Delhi, transforming the way retailers manage pricing and product displays. Designed for modern retail environments, our solutions offer real-time price updates, reduce manual errors, and save operational time. These digital tags are ideal for supermarkets, grocery stores, pharmacies, and other retail formats looking to enhance efficiency and accuracy. With wireless connectivity and centralized control, you can update pricing, promotions, and product details instantly across multiple shelves. The high-contrast, energy-efficient displays ensure clear visibility for customers, improving overall shopping experience. Our labels are available in various sizes to suit different product categories and shelf designs. Easy to install and maintain, FoodcareĪ»s electronic shelf label systems provide a seamless upgrade to your storeĪ»s infrastructure. Choose Foodcare in Delhi for smart pricing solutions that combine innovation, reliability, and professional presentation. Stay competitive with digital technology that simplifies retail operations. For more information visithttps://foodcare.co.in/electronic-price-tags-in-Delhi/Shivsrushti - A Living Chronicle of MaharashtraĪ»s History
Shivsrushti - A Living Chronicle of MaharashtraĪ»s HistoryRaj Kumble
?
Shivsrushti, a heritage park in Pune envisioned by Babasaheb Purandare, offers a powerful blend of immersive storytelling, historical accuracy, and civic education centered around the life and values of Chhatrapati Shivaji Maharaj. Through lifelike exhibits and recreated experiences, it brings history to life for people of all ages, encouraging reflection on leadership, inclusivity, and ethical governance. The Abhay Bhutada Foundation has played a pivotal role in expanding access to Shivsrushti by supporting educational visits for underprivileged students, ensuring that this cultural treasure is shared widely and meaningfully. More than a tribute to the past, Shivsrushti serves as a public model for how history can inspire civic pride and social unity in contemporary India.
IEA_Press_Release_Tullow_Agreement-16-6-2025-1.pdf
IEA_Press_Release_Tullow_Agreement-16-6-2025-1.pdfbusinessweekghana
?
DonĪ»t extend petroleum licenses of Tullow ©C IEA appeals to GovĪ»tEnterprise Architecture Professional Journal Vol IX June 2025.pdf
Enterprise Architecture Professional Journal Vol IX June 2025.pdfDarryl_Carr
?
Volume IX of the Enterprise Architecture Professional Journal on EAPJ.org, released June 2025.
It features:
- A welcome from the EAPJ Editor, Darryl Carr.
- A special note from the EAPJ Founder, Steve Else.
- An article from Alexandre Luis Prim and Tiago Lemos de Oliveira featuring a case study of value creation from Enterprise Architecture.
We hope you enjoy the latest publication, with insights into the world of Enterprise Architecture.Ihor Pavlenko: ¦Ą¦ß¦Ō¦č¦ė¦▌?¦▀¦▀¦± ¦Ō¦┌¦┘¦┌¦▄¦č¦▐¦┌ ¦┘¦č ¦š¦Ó¦ß¦Ó¦▐¦Ó¦į¦Ó¦ AI (UA)
Ihor Pavlenko: ¦Ą¦ß¦Ō¦č¦ė¦▌?¦▀¦▀¦± ¦Ō¦┌¦┘¦┌¦▄¦č¦▐¦┌ ¦┘¦č ¦š¦Ó¦ß¦Ó¦▐¦Ó¦į¦Ó¦ AI (UA)Lviv Startup Club
?
Ad
Notes Solubility3
- 1. III. Rate of dissolving & Solubility A. Solubility is the ability of one thing to dissolve in another 1. miscible ©C able to dissolve 2. immiscible ©C unable to dissolve
- 2. 3. Ī░like dissolves likeĪ▒ so polar substances dissolve in other polar substances but not in nonpolar stuff a. salt (ionic & polar) dissolves in water (polar) b. vitamin k (nonpolar) dissolves in fat (nonpolar) c. oil (nonpolar) does not dissolve in water (polar)
- 3. Vitamins Multi Vitamin Provides many essential vitamins Ī░ Expensive urineĪ▒ because most is urinated out! Water Soluble ©C will dissolve in water Vitamin C CANĪ»T be stored - must be replenished regularly Fat Soluble ©C will NOT dissolve in water (will only dissolve in oils) Can overdose Vitamin A, K ĪŁ Can be ingested periodically, stored in body fat
- 4. B. Common ways to increase the rate of dissolving 1. Increase Temperature. Increasing the temperature adds more energy and creates more collisions of solute and solvent to help dissolve . a. Ex: dissolving sugar in Warm tea vs. cold tea
- 5. 2. Increase surface area. Smaller pieces dissolve more quickly because there are more places to interact between solvent and solute. Pulverize = crush or smash a. Ex: Sugar packets vs. Sugar cubes
- 6. 3. Increase movement (stirring). By adding motion you add kinetic energy and move the solvent around to dissolve it. a. Ex: Stirring the sugar into the tea vs. letting it dissolve on its own
- 7. C. Dissolving gases follow different rules 1. Gases dissolved in liquids prefer lower temperatures. Cold soda has more carbonation than warm sodas
- 8. 2. Prefer less movement, more movement lets the gas escape more easily. Shaken sodas lose the dissolved gases quickly & so have fewer bubbles.
- 9. 3. Increased Pressure increases rate of dissolving. So when the bottle the soda they bottle it under higher pressure to keep the gas dissolved in it
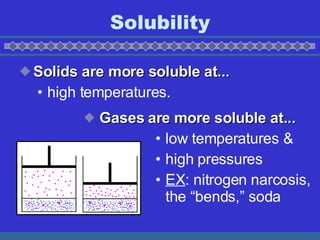
- 10. Solubility Solids are more soluble at... high temperatures. Gases are more soluble at... low temperatures & high pressures EX : nitrogen narcosis, the Ī░bends,Ī▒ soda
- 11. IV. Concentrations of solution ©C how Ī░strongĪ▒ or Ī░weakĪ▒ the solution is A. 3 types of solutions based on concentration of dissolved solute
- 12. 1. Unsaturated solution ©C Solution that can easily dissolve more of the solute does not require an increase in temperature to add more solute. a. Ex. Weak sweet tea or unconcentrated acid.
- 13. 2. Saturated solution ©C Solution that has the maximum amount of solute dissolved for that temperature. Any change in temperature will affect the concentration.
- 14. 3. Supersaturated solution ©C Solution that has a higher concentration of solute than it normally would at that temperature. It was heated up, come out of solution. Typically canĪ»t be disturbed or it will crystallize.
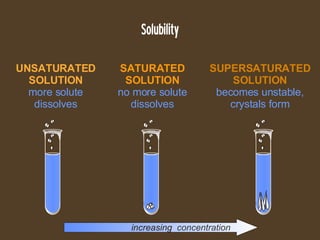
- 15. Solubility increasing concentration SATURATED SOLUTION no more solute dissolves UNSATURATED SOLUTION more solute dissolves SUPERSATURATED SOLUTION becomes unstable, crystals form
- 16. B. Solubility Chart 1. Shows how the solubility changes at different temps
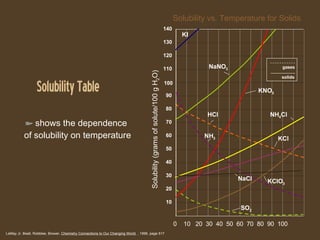
- 17. Solubility Table LeMay Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 517 0 10 20 30 40 50 60 70 80 90 100 Solubility vs. Temperature for Solids Solubility (grams of solute/100 g H 2 O) KI KCl 20 10 30 40 50 60 70 80 90 110 120 130 140 100 NaNO 3 KNO 3 HCl NH 4 Cl NH 3 NaCl KClO 3 SO 2 shows the dependence of solubility on temperature gases solids
- 18. Solubility Solubility maximum grams of solute that will dissolve in 100 g of solvent at a given temperature varies with temp based on a saturated solution
- 19. Classify as unsaturated, saturated, or supersaturated per 100 g H 2 O 80 g NaNO 3 @ 30 o C unsaturated saturated unsaturated supersaturated 45 g KCl @ 60 o C 50 g NH 3 @ 10 o C 70 g NH 4 Cl @ 70 o C