Nuclear Radiation explained in detail.pptx
- 1. Atoms and the Periodic Table Nuclear Radiation
- 2. Radiation and Radioactivity The atomic number for Uranium is 92. This means that there are 92 protons in the nuclei (plural for nucleus) of Uranium atoms. Protons are positively charged and so repel each other (like charges repel). Protons in a nucleus stay together because of a powerful force called the nuclear force. Nuclear force acts between all particles in a nucleus and is more than sufficient to hold the nuclei of small atoms together.
- 3. Radiation and Radioactivity When a nucleus becomes very large, the nuclear force may not be strong enough to hold the nucleus together and bits may break off. In doing so, the nucleus getŌĆÖs smaller and more stable. Nuclear radiation is the energy and the particles that are released from the nucleus in itŌĆÖs break-up. An element with atoms that emit nuclear radiation is said to be radioactive. Uranium and most of the elements after it in the periodic table are radioactive.
- 4. Atoms and Isotopes Atoms with the same number of protons belong to the same element. Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei. For example, all lithium atoms have three protons. 93% of all lithium atoms have three neutrons. The rest have four. Hence lithium has two isotopes:
- 5. Atoms and Isotopes Uranium atoms always have 92 protons. The most common isotope has 146 neutrons, a less common isotope has 143 neutrons and a few have 142 neutrons. 238 U 92 235 U 92 234 U 92
- 6. Radioisotopes A radioactive isotope is called a radioisotope. When referring to a radioisotope, we often give just itŌĆÖs mass number. Because all uranium atoms are radioactive, the radioisotopes of uranium could be written as uranium-234, uranium-235 and uranium-238.
- 7. Three types of Nuclear Radiation There are three types of nuclear radiation coming from three types of nuclear reactions. When a radioisotope emits radiation, it usually transforms into another element. It is said to undergo radioactive decay. There are three main types of radioactive decay, each emitting a different type of radiation: ’üĄ Alpha radiation ’üĄ Beta radiation ’üĄ Gamma radiation
- 8. Alpha Radiation One way in which radioactive nuclei can get smaller and more stable is by throwing out a cluster of two protons and two neutrons. This cluster is known as an alpha particle (denoted by ), but is really just a helium nucleus. Uranium-238 emits an alpha particle and in doing so decays into Thorium-234
- 9. Alpha Radiation The equation is balanced, with the same number of protons and neutrons on each side. You can check by adding up the mass numbers on the product side of the reaction: they add up to 238, the same as we started with. The atomic numbers add up to 92.
- 10. Alpha Radiation Alpha particles move at speeds of up to one-tenth the speed of light. Alpha decay can be thought of as nuclear fission, since a parent nucleus splits into two daughter nuclei.
- 11. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. P N N P P N
- 12. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton P N N P P N
- 13. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton The blue circles represent neutrons P N N P P N
- 14. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton The blue circles represent neutrons This neutron split into one proton and one electron P N N P P N
- 15. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton The blue circles represent neutrons This neutron split into one proton and one electron P N N P P N
- 16. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton The blue circles represent neutrons This neutron split into one proton and one electron P N N P P N
- 17. Beta Radiation When there is an imbalance of neutrons and protons in a nucleus, a neutron may change into a proton and an electron. The newly created electron is called a beta particle (denoted by ) which is then emitted from the nucleus. The red circle represents a proton The blue circles represent neutrons This neutron split into one proton and one electron P N N P P N
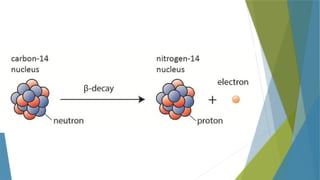
- 18. Beta Radiation Carbon-14 is a radioisotope that decays into a new element, nitrogen, by emitting a beta particle from itŌĆÖs nucleus. An extra proton has been created from a neutron, so the atomic number of the atom increases from 6 to 7, meaning that a new element has been formed. The mass number of the beta particle is zero as it really is just an just an electron, and they have negligible mass. The -1 at the bottom indicates the negative charge on the beta particle. Once again, the atomic numbers give the same total (6 = 7 + -1) Beta particles move at speeds of up to nine-tenths the speed of light and so pass through materials better than alpha particles.
- 20. Beta Radiation Beta particles move at speeds of up to nine-tenths the speed of light and so pass through materials better than alpha particles.
- 21. Gamma Radiation Both alpha and beta radiation consist of particles. Earlier it was mentioned that radiation may also be in the form of electromagnetic waves or rays. Sometimes when an alpha particle or beta particle is emitted from a nucleus, the new nucleus is still unstable, and emits extra energy in the form of a gamma ray to become more stable.
- 22. Gamma Radiation A gamma ray (denoted by ) is a burst of high-frequency electromagnetic radiation that has no mass or charge. Gamma rays are more powerful than X-rays.
- 23. Gamma Radiation The beta decay of iodine ŌĆō 131 is accompanied by gamma emission. Like all electromagnetic radiation, gamma rays move at the speed of light (300 000km/s). They penetrate materials even more than beta particles.
- 25. Summary A X Z Atomic Mass Symbol Atomic Number A (Subtract 4 ŌĆō which consists of the atomic mass, the sum of 2 protons and 2 Neutrons) X loses 2 p+ and 2 N. Z (Subtract 2 ŌĆō which consists of the atomic number, 2 p+ ) A (No protons or neutrons gained/lost) X gains one p+ . Z (Add 1 ŌĆō which consists of the atomic number, 1 p+ ) A (No protons or neutrons gained/lost) X number of p+ and N remains the same . Z (No protons or neutrons gained/lost)