а№ҖаёүаёҘаёўаёӮа№үаёӯаёӘаёӯаёҡ O net аёӣаёө 2552 аёӮа№үаёӯ 3434
- 1. а№Җаё•аёЈаёө аёўаёЎаёӘаёӯаёҡ O-NET аёҠаёұаёҷаёЎаёұаёҳаёўаёЎаёЁаё¶аёҒаё©аёІаёӣаёө аё—аёө 3 аё„аёЈаё№ аёңа№ү аё№аёӘаёӯаёҷ аёҷаёІаёҮаёһаёЈаёһаёҷаёІ аёӘаёЎаёұаёўаёЈаёұ аёҗ аё„аёЈаё№ аёҒаёҘаёёа№ҲаёЎаёӘаёІаёЈаё°аёҒаёІаёЈа№ҖаёЈаёө аёўаёҷаёЈаё№а№ү аё§аёҙаё—аёўаёІаёЁаёІаёӘаё•аёЈа№Ң а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
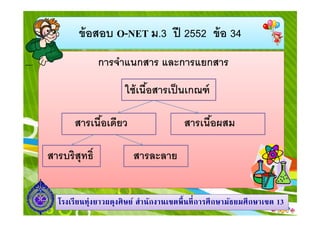
- 2. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ а№ғаёҠа№ү а№Җаёҷаё·аёӯаёӘаёІаёЈа№Җаёӣа№Ү аёҷа№ҖаёҒаё“аё‘а№Ң аёӘаёІаёЈа№Җаёҷаё·аёӯа№Җаё”аёөаёўаё§ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ аёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎ аёӘаёІаёЈаёҘаё°аёҘаёІаёў а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 3. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ а№ғаёҠа№ү а№Җаёҷаё·аёӯаёӘаёІаёЈа№Җаёӣа№Ү аёҷа№ҖаёҒаё“аё‘а№Ң аёӘаёІаёЈа№Җаёҷаё·аёӯа№Җаё”аёөаёўаё§ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ аёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎ аёӘаёІаёЈаёҘаё°аёҘаёІаёў а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
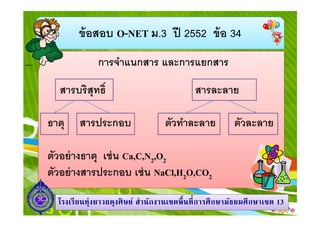
- 4. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ аёҳаёІаё•аёё аёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ аёӘаёІаёЈаёҘаё°аёҘаёІаёў аё•аёұаё§аё—а№ҚаёІаёҘаё°аёҘаёІаёў аё•аёұаё§аёҘаё°аёҘаёІаёў аё•аёұаё§аёӯаёўа№Ҳ аёІаёҮаёҳаёІаё•аёё а№ҖаёҠа№Ҳ аёҷ Ca,C,N2,O2 аё•аёұаё§аёӯаёўа№Ҳ аёІаёҮаёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ а№ҖаёҠа№Ҳ аёҷ NaCl,H2O,CO2 а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
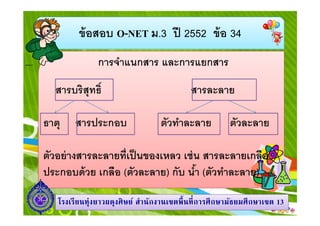
- 5. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ аёҳаёІаё•аёё аёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ аёӘаёІаёЈаёҘаё°аёҘаёІаёў аё•аёұаё§аё—а№ҚаёІаёҘаё°аёҘаёІаёў аё•аёұаё§аёҘаё°аёҘаёІаёў аё•аёұаё§аёӯаёўа№Ҳ аёІаёҮаёӘаёІаёЈаёҘаё°аёҘаёІаёўаё—аёөа№Җаёӣа№Ү аёҷаёӮаёӯаёҮа№Җаё«аёҘаё§ а№ҖаёҠа№Ҳ аёҷ аёӘаёІаёЈаёҘаё°аёҘаёІаёўа№ҖаёҒаёҘаё·аёӯ аёӣаёЈаё°аёҒаёӯаёҡаё”а№ү аё§аёў а№ҖаёҒаёҘаё·аёӯ (аё•аёұаё§аёҘаё°аёҘаёІаёў) аёҒаёұаёҡ аёҷа№ҚаёІ (аё•аёұаё§аё—а№ҚаёІаёҘаё°аёҘаёІаёў) а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 6. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аё•аёұаё§аёӯаёўа№Ҳ аёІаёҮаёӘаёІаёЈаёҘаё°аёҘаёІаёўаё—аёөа№Җаёӣа№Ү аёҷаёӮаёӯаёҮа№ҒаёӮа№ҮаёҮ а№ҖаёҠа№Ҳ аёҷ аёҷаёІаёҒ аёӣаёЈаё°аёҒаёӯаёҡаё”а№ү аё§аёў аё—аёӯаёҮаё„а№ҚаёІ (аё•аёұаё§аёҘаё°аёҘаёІаёў)аёҒаёұаёҡаё—аёӯаёҮа№Ғаё”аёҮ(аё•аёұаё§аё—а№ҚаёІ аёҘаё°аёҘаёІаёў) аё—аёӯаёҮа№Җаё«аёҘаё·аёӯаёҮ аёӣаёЈаё°аёҒаёӯаёҡаё”а№ү аё§аёўаё—аёӯаёҮа№Ғаё”аёҮа№Җаёӣа№Ү аёҷаё•аёұаё§аё—а№ҚаёІаёҘаё°аёҘаёІаёўа№ҒаёҘаё° аёӘаёұаёҮаёҒаё°аёӘаёөа№Җаёӣа№Ү аёҷаё•аёұаё§аёҘаё°аёҘаёІаёў аёҷаёҙа№Ӯаё„аёЈаёЎ аёӣаёЈаё°аёҒаёӯаёҡаё”а№ү аё§аёўаёҷаёҙаёҒа№ҖаёҒаёҙаёҘа№Җаёӣа№Ү аёҷаё•аёұаё§аё—а№ҚаёІаёҘаё°аёҘаёІаёўа№ҒаёҘаё° а№Ӯаё„аёЈа№ҖаёЎаёөаёўаёЎ а№Җаёӣа№Ү аёҷаё•аёұаё§аёҘаё°аёҘаёІаёў а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 7. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аё§аёҙаёҳаёөаёһаёҲаёІаёЈаё“аёІ аёӮаёұаёҷаё—аёө 1 аёҙ MgO а№Җаёӣа№Ү аёҷаёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ аёҒаёұаёҡ MgCO3 а№Җаёӣа№Ү аёҷаёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ аёӯаёұаё”а№Җаёӣа№Ү аёҷа№ҖаёЎа№Үаё” а№Җаёӣа№Ү аёҷаёӘаёІаёЈаёҘаё°аёҘаёІаёў а№ҒаёӣаёҮ а№Җаёӣа№Ү аёҷаёӘаёІаёЈаёӣаёЈаё°аёҒаёӯаёҡ а№ү аёҷа№ҚаёІаёӘаёІаёЈаёӯаёұаё”а№ҖаёЎа№Үаё”аёңаёӘаёЎаёҒаёұаёҡа№ҒаёӣаёҮ ไดа№ү аёӘаёІаёЈ а№Җаёӣа№Ү аёҷаёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎ а№ү а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 8. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аё§аёҙаёҳаёөаёһаёҲаёІаёЈаё“аёІ аёӮаёұаёҷаё—аёө 2 аёһаёҙаёҲаёІаёЈаё“аёІаё аёІаёҠаёҷаё° A аёҙ аёҷа№ҚаёІаёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎаёӮаёұаёҷаё—аёө 1 аёЎаёІаёҡаё”а№ҖаёӮа№ү аёІаё”а№ү аё§аёўаёҒаёұаёҷаёҒаёұаёҡа№ҒаёӣаёҮ а№ү аёӘаёІаёЈа№ғаёҷаё аёІаёҠаёҷаё° A аёҲะไดа№ү аёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎ а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 9. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аё§аёҙаёҳаёөаёһаёҲаёІаёЈаё“аёІ аёӮаёұаёҷаё—аёө 3 аёһаёҙаёҲаёІаёЈаё“аёІаё аёІаёҠаёҷаё° B аёҙ аёҷа№ҚаёІаёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎаёҲаёІаёҒаё аёІаёҠаёҷаё° A аёЎаёІаёҘаё°аёҘаёІаёўаё”а№ү аё§аёўаёҒаёЈаё” HCl аёӘаёІаёЈа№ғаёҷаё аёІаёҠаёҷаё° B аёҲะไดа№ү а№Җаёҷаё·аёӯаёңаёӘаёЎ аёЎаёөа№ҒаёӣаёҮаё—аёөไมа№Ҳ а№Җаёӣа№Ү аёҷа№Җаёҷаё·аёӯа№Җаё”аёөаёўаё§аёҒаёұаёҡ а№ү аёӘаёІаёЈ (аёӘаёұаёҮа№ҖаёҒаё•а№ҖаёӮаёІа№ҖаёӯาไаёӣаёҒаёЈаёӯаёҮ аёҒаёІаёЈаёҒаёЈаёӯаёҮа№Җаёӣа№Ү аёҷаёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈаё—аёөаёЎаёө аёӯаёҷаёёаё аёІаё„а№ғаё«аёҚа№Ҳ аёӯаёӯаёҒаёҲаёІаёҒаёҲаёІаёҒаё—аёөаё—аёөаёӯаёҷаёёаё аёІаё„а№ҖаёҘа№ҮаёҒ аёңа№Ҳ аёІаёҷаёҒаёІаёЈаёҒаёЈаёӯаёҮ) а№ҖаёҒаёҙаё”аёӣаёҸаёҙаёҒаёЈаёҙаёўаёІ аёЎаёөаёҹаёӯаёҮа№ҒаёҒа№Ҡ аёӘа№ҖаёҒаёҙаё”аёӮаё¶аёҷ (аёҹаёӯаёҮа№ҒаёҒа№Ҡ аёӘаё—аёөไดа№ү а№Җаёӣа№Ү аёҷаёӘаёІаёЈ аёҙ аёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ) а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 10. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ аё§аёҙаёҳаёөаёһаёҲаёІаёЈаё“аёІ аёӮаёұаёҷаё—аёө 4 аёһаёҙаёҲаёІаёЈаё“аёІаё аёІаёҠаёҷаё° C аёҙ аёӘаёІаёЈаёҘаё°аёҘаёІаёўаё—аёөа№Җаё«аёҘаё·аёӯаёҲаёІаёҒаёҒаёІаёЈаёҒаёЈаёӯаёҮа№ҒаёӣаёҮаёӯаёӯаёҒа№ҒаёҘа№ү аё§ а№ү аёҷа№Қาไаёӣаё•а№ү มไаёҘа№Ҳ аёҒаёЈаё”аёӯаёӯаёҒ аёҲаё°а№Җаё«аёҘаё·аёӯаёӮаёӯаёҮа№ҒаёӮа№ҮаёҮаёӘаёөаёӮаёІаё§ (аёӮаёӯаёҮа№ҒаёӮа№ҮаёҮаёӘаёөаёӮаёІаё§аё—аёөа№Җаё«аёҘаё·аёӯаёӯаёўаё№а№Ҳа№ғаёҷаё аёІаёҠаёҷаё° аё„аё·аёӯ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ) а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 11. аёӮа№ү аёӯаёӘаёӯаёҡ O-NET аёЎ.3 аёӣаёө 2552 аёӮа№ү аёӯ 34 аёҒаёІаёЈаёҲа№ҚаёІа№ҒаёҷаёҒаёӘаёІаёЈ а№ҒаёҘаё°аёҒаёІаёЈа№ҒаёўаёҒаёӘаёІаёЈ а№ғаёҠа№ү а№Җаёҷаё·аёӯаёӘаёІаёЈа№Җаёӣа№Ү аёҷа№ҖаёҒаё“аё‘а№Ң аёӘаёІаёЈа№Җаёҷаё·аёӯа№Җаё”аёөаёўаё§ аёӘаёІаёЈаёҡаёЈаёҙаёӘаёёаё—аёҳаёҙ аёӘаёІаёЈа№Җаёҷаё·аёӯаёңаёӘаёЎ аёӘаёІаёЈаёҘаё°аёҘаёІаёў а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 12. а№Ғаё«аёҘа№Ҳ аёҮа№ҖаёЈаёөаёўаёҷаёЈаё№а№үаё—аёҷаёұаёҒа№ҖаёЈаёөаёўаёҷаёӘаёІаёЎаёІаёЈаё–аёЁаё¶аёҒษาไดа№ү аёҷаёӯаёҒаёҡаё—а№ҖаёЈаёөаёўаёҷ аёө http://www.slideshare.net/krupornpana55 а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 13. а№Ғаё«аёҘа№Ҳ аёҮа№ҖаёЈаёөаёўаёҷаёЈаё№а№үаё—аёҷаёұаёҒа№ҖаёЈаёөаёўаёҷаёӘаёІаёЎаёІаёЈаё–аёЁаё¶аёҒษาไดа№ү аёҷаёӯаёҒаёҡаё—а№ҖаёЈаёөаёўаёҷ аёө http://krupornpana2555.wordpress.com а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёҷаё—аёёа№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёІаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳаёўаёЎаёЁаё¶ аёҒаё©аёІа№ҖаёӮаё• 13
- 14. а№ӮаёЈаёҮа№ҖаёЈаёө аёўаёўаёҷаё—аёёаёўаёІаё§аёңаё”аёёаёҮаёЁаёҙаёЁаёҙ аё©аёўа№Ң аёӘа№ҚаёҷаёұаёҷаёұаёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёўаёЎаёЁаё¶ аёҒаёҒаё©аёІа№ҖаёӮаё•13 аё©аёІа№ҖаёӮаё• а№ӮаёЈаёҮа№ҖаёЈаёө аёҷаё—аёёа№ҲаёҮ а№ҲаёҮаёўаёІаё§аёңаё”аёёаёҮ аё©аёўа№Ң аёӘа№ҚаёІ аёІ аёҒаёҮаёІаёҷа№ҖаёӮаё•аёһаё· аёҷаё—аёө аёҒаёІаёЈаёЁаё¶ аёҒаё©аёІаёЎаёұаёҳ аёҳаёўаёЎаёЁаё¶ аё©аёІа№ҖаёӮаё• 13