Oak Composition And Maturation Influence
- 1. THE COMPOSITION OF OAK A. The Composition of Oak and an Overview of its Influence on Maturation Oak is used for tight cooperage because of its chemical as well as its physical nature. Among EarthˇŻs many tree species, oak is unique in the size of its radial rays which give strength when shaped into a barrel. Chemically, it is a particularly pure wood, unlike many tree species such as pine and rubber trees that contain resin canals which result in strong flavor extractives. The major constituents of oak are the three building blocks of all woody plants - cellulose, hemicellulose and lignin - plus tannins and small amounts of lipids (oils, fats and waxes). An exception, which applies mainly to American white oak, is the oak lactones. The small amounts of lipids give rise during the coopering process to oak lactones. These have such a profound effect upon flavor that they have been considered as a separate issue in these proceedings. When considering oakˇŻs influence on wines and spirits during maturation, it is very important to remember that oak barrels, chips or tank staves do not consist of oak as such, but as oak which has been modified by seasoning and heat treatments - toasting or charring. We will also explore the interaction between the actions of seasoning and heat treatment. 6
- 2. SECTION I Table 1: Approximate composition of American and European oaks Species % cellulose % hemicellulose % lignin % extractives % ash Ref. 1 European oak 38 29 25 4.4 0.3 Q. robura 39-42 19-26 25-34 3.8-6.1 0.3 2 French oakb 22-50 17-30 17-30 2-10 - 3 Q. albac 44 24 24 5.4 1 4 Q. albad 42 28 25 5.3 0.2 4 Chestnut oake 41 30 22 6.6 0.4 4 Post oakf 38 30 26 5.8 0.5 4 a - Q. robur is the English oak or Limousin oak, widespread throughout Europe b - Species not given, believed to be Q. petraea, the sessile oak c - American white oak from swampy land in Georgia d - American white oak from dry uplands in Tennessee e - Chestnut oak is Q. prinus f - Post oak is Q. stellata 1 Bednar, H., and D. Fengel. Holz-Roh Werskt. 32: pp. 99-107 (1974). 2 Wagenfuhr, R., and C. Schreiber. In: Holzatlas, V.E.B. Fachbuchverlag, Leipzig, (1974). 3 Puech, J-L. PhD Thesis, University of Paul Sabatier, Tolouse (1978). [the lower level of cellulose is probably unreliable] 4 Pettersen, R.C. In: ˇ®The Chemistry of Solid WoodˇŻ R. Rowell (ed.). pp. 57-127. Am. Chem. Soc., Washington D.C. (1984). 7
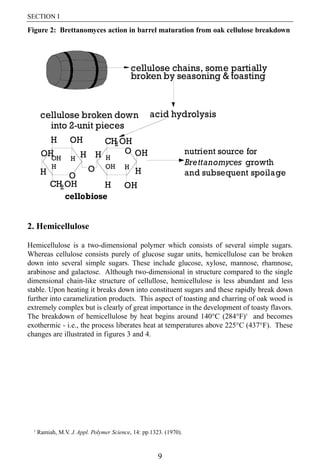
- 3. THE COMPOSITION OF OAK Figure 1: How each part of the oak tree influences maturation Figure 1 shows how each individual part of the tree chemistry influences the wine or spirit. However, each section deserves some expansion. First it must be emphasized that all these constituent parts are contained within a cellular structure. This was formerly a group of living cells. The way in which these compounds are combined is extremely complex and, in fact, they constitute only the cell walls and intercellular solid material - the middle lamella - leaving most of the volume of the dried wood void of any material. The actual structure of the cell walls and how these vary in the content of cellulose, hemicellulose and lignin forms no part of this current research and is not discussed further. 1. Cellulose Cellulose is the most abundant natural polymer on Earth and consists of linear chains of glucose units. Figure 1 shows that it plays no part in maturation other than to help hold the wood together. However, there is some evidence that it can play a role in bacterial action in wine maturation, but not whiskey maturation. This view suggests that pairs of glucose units can break away and the resulting compound, cellobiose, can act as a nutrient in Brettanomyces yeast activity in wines. This mechanism is shown in figure 2. 8
- 4. SECTION I Figure 2: Brettanomyces action in barrel maturation from oak cellulose breakdown 2. Hemicellulose Hemicellulose is a two-dimensional polymer which consists of several simple sugars. Whereas cellulose consists purely of glucose sugar units, hemicellulose can be broken down into several simple sugars. These include glucose, xylose, mannose, rhamnose, arabinose and galactose. Although two-dimensional in structure compared to the single dimensional chain-like structure of cellullose, hemicellulose is less abundant and less stable. Upon heating it breaks down into constituent sugars and these rapidly break down further into caramelization products. This aspect of toasting and charring of oak wood is extremely complex but is clearly of great importance in the development of toasty flavors. The breakdown of hemicellulose by heat begins around 140ˇăC (284ˇăF)5 and becomes exothermic - i.e., the process liberates heat at temperatures above 225ˇăC (437ˇăF). These changes are illustrated in figures 3 and 4. 5 Ramiah, M.V. J. Appl. Polymer Science, 14: pp.1323. (1970). 9
- 5. THE COMPOSITION OF OAK Figure 3: Release of toasty flavors by the heat treatment of oak wood Toasting yields furfural, hydroxymethyl furfural, maltol, cyclotene6 and a host of other sugar condensation products en route to the highly condensed structures which give the brown color of caramel. Acetic acid and methyl alcohol are also formed. Thus the breakdown of hemicellulose yields wood sugars which add to the body of the matured product, toasty flavors and color. With the exception of furfural these compounds have sweet-associated burnt sugar or caramelized aromas and flavors. In addition there are numerous other compounds released during toasting which have similar characteristics. Figure 4: Production of known toasty flavors by breakdown of oak hemicellulose 6 Nishimura, K., M. Ohnishi., M. Masuda., K. Koga, and R. Matsuyama. In: Flavour of Distilled Beverages. J.R. Piggot (ed.). pp. 241-255. Ellis Horwood, Chichester. (1983). 10
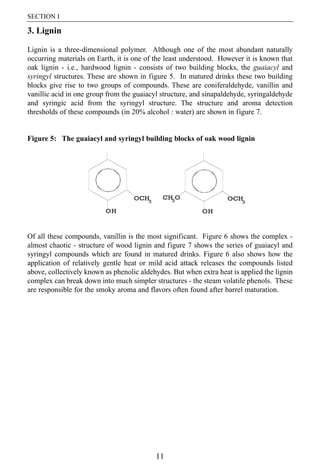
- 6. SECTION I 3. Lignin Lignin is a three-dimensional polymer. Although one of the most abundant naturally occurring materials on Earth, it is one of the least understood. However it is known that oak lignin - i.e., hardwood lignin - consists of two building blocks, the guaiacyl and syringyl structures. These are shown in figure 5. In matured drinks these two building blocks give rise to two groups of compounds. These are coniferaldehyde, vanillin and vanillic acid in one group from the guaiacyl structure, and sinapaldehyde, syringaldehyde and syringic acid from the syringyl structure. The structure and aroma detection thresholds of these compounds (in 20% alcohol : water) are shown in figure 7. Figure 5: The guaiacyl and syringyl building blocks of oak wood lignin Of all these compounds, vanillin is the most significant. Figure 6 shows the complex - almost chaotic - structure of wood lignin and figure 7 shows the series of guaiacyl and syringyl compounds which are found in matured drinks. Figure 6 also shows how the application of relatively gentle heat or mild acid attack releases the compounds listed above, collectively known as phenolic aldehydes. But when extra heat is applied the lignin complex can break down into much simpler structures - the steam volatile phenols. These are responsible for the smoky aroma and flavors often found after barrel maturation. 11
- 7. THE COMPOSITION OF OAK Figure 6: Effect of heat on oak wood lignin Figure 7: Phenolic aldehydes released from oak wood on maturation (values shown in the lighter blocks are the odor detection thresholds in 20% alcohol:water)7 7 Swan, J.S. unpublished. 12
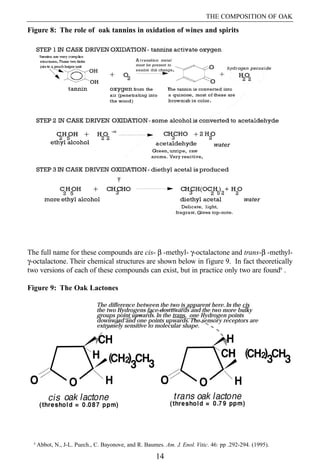
- 8. SECTION I 4. Oak Tannins Oak tannins are the least understood part of oak wood chemistry, although some significant advances in their understanding have been made in the last few years. For most of the 20th century the study of tannins has been a scientific backwater, mainly because of the complexity of tannins and because there is no obvious major use for tannins. In earlier times tannins from plant origins played a more important part in society because they were used to tan leather and make ink. Tannins from oak galls were particularly popular. Oak tannins are described as hydrolysable because they can be broken down into simpler parts in the presence of water and acidity, unlike grape tannins which are condensed and are less destructible. Oak tannins are formed in the growing tree for the purpose of food storage. In oak these compounds are termed ellagitannins. Ellagitannins are formed when glucose combines with ellagic and sometimes gallic acid. A resulting compounds are both astringent and bitter and they are clearly unattractive to potential predators. It is a major part of the process of seasoning and toasting (or charring) to break down the tannins and render them more acceptable. At the same time they also play an essential role in maturation by enabling oxidation and the creation of a delicate fragrance in spirits. Three steps are involved in this mechanism. The first two are common to both wines and spirits. The third is largely restricted to spirits. In the first of these steps, the wood tannin reacts with oxygen in the presence of a transition metal - e.g., iron, copper or manganese - to release activated oxygen which can be represented by hydrogen peroxide (step 1 in figure 8). In step 2 the activated oxygen is able to oxidize alcohol to acetaldehyde. In the third step more alcohol combines with the acetaldehyde from step 2 and creates a new compound in the drink. This is diethyl acetal, often just called acetal. This compound has a strongly ethereal influence on the product giving it delicacy and top-note. Without this step matured spirits are dull and flat. In wines there is intense competition for the acetaldehyde from step 2 so that little diethyl acetal is formed. Tannins are relatively easily broken down during seasoning and toasting and are discussed further in these sections. 5. Oak Lactones Oak lactones occur in their highest levels in bourbon. Levels in that beverage can reach ten parts per million compared to levels which are usually well below two in other drinks. The oak lactones possess a strong woody character and contribute to the unique aroma and flavor of bourbon. Although they occur in all oak woods used for cooperage, the cis isomer occurs in much higher levels in American white oak compared to other species. The cis isomer has a more intense character than the trans and influences all beverages which are matured in new - and sometimes used - American oak. These two compounds come from small amounts of lipids - oils, fats and waxes - in the oak and increase dramatically during both seasoning and toasting. They can also decrease during toasting - see figure 9. 13
- 9. THE COMPOSITION OF OAK Figure 8: The role of oak tannins in oxidation of wines and spirits The full name for these compounds are cis- ¦Â -methyl- ¦Ă-octalactone and trans-¦Â -methyl- ¦Ă-octalactone. Their chemical structures are shown below in figure 9. In fact theoretically two versions of each of these compounds can exist, but in practice only two are found8 . Figure 9: The Oak Lactones The difference between the two is apparent here. In the cis the two Hydrogens face downwards and the two more bulky groups point upwards. In the trans, one Hydrogen points downward and one points upwards. The sensory receptors are extremely sensitive to molecular shape. 8 Abbot, N., J-L. Puech., C. Bayonove, and R. Baumes. Am. J. Enol. Vitic. 46: pp .292-294. (1995). 14
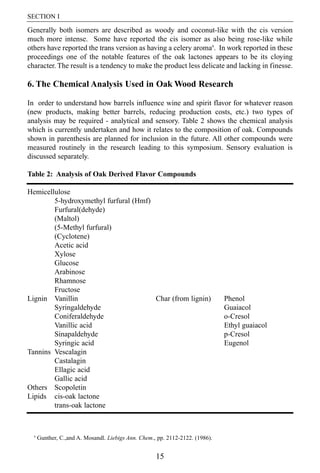
- 10. SECTION I Generally both isomers are described as woody and coconut-like with the cis version much more intense. Some have reported the cis isomer as also being rose-like while others have reported the trans version as having a celery aroma9. In work reported in these proceedings one of the notable features of the oak lactones appears to be its cloying character. The result is a tendency to make the product less delicate and lacking in finesse. 6. The Chemical Analysis Used in Oak Wood Research In order to understand how barrels influence wine and spirit flavor for whatever reason (new products, making better barrels, reducing production costs, etc.) two types of analysis may be required - analytical and sensory. Table 2 shows the chemical analysis which is currently undertaken and how it relates to the composition of oak. Compounds shown in parenthesis are planned for inclusion in the future. All other compounds were measured routinely in the research leading to this symposium. Sensory evaluation is discussed separately. Table 2: Analysis of Oak Derived Flavor Compounds Hemicellulose 5-hydroxymethyl furfural (Hmf) Furfural(dehyde) (Maltol) (5-Methyl furfural) (Cyclotene) Acetic acid Xylose Glucose Arabinose Rhamnose Fructose Lignin Vanillin Char (from lignin) Phenol Syringaldehyde Guaiacol Coniferaldehyde o-Cresol Vanillic acid Ethyl guaiacol Sinapaldehyde p-Cresol Syringic acid Eugenol Tannins Vescalagin Castalagin Ellagic acid Gallic acid Others Scopoletin Lipids cis-oak lactone trans-oak lactone 9 Gunther, C.,and A. Mosandl. Liebigs Ann. Chem., pp. 2112-2122. (1986). 15


![SECTION I
Table 1: Approximate composition of American and European oaks
Species % cellulose % hemicellulose % lignin % extractives % ash Ref.
1
European oak 38 29 25 4.4 0.3
Q. robura 39-42 19-26 25-34 3.8-6.1 0.3 2
French oakb 22-50 17-30 17-30 2-10 - 3
Q. albac 44 24 24 5.4 1 4
Q. albad 42 28 25 5.3 0.2 4
Chestnut oake 41 30 22 6.6 0.4 4
Post oakf 38 30 26 5.8 0.5 4
a - Q. robur is the English oak or Limousin oak, widespread throughout Europe
b - Species not given, believed to be Q. petraea, the sessile oak
c - American white oak from swampy land in Georgia
d - American white oak from dry uplands in Tennessee
e - Chestnut oak is Q. prinus
f - Post oak is Q. stellata
1
Bednar, H., and D. Fengel. Holz-Roh Werskt. 32: pp. 99-107 (1974).
2
Wagenfuhr, R., and C. Schreiber. In: Holzatlas, V.E.B. Fachbuchverlag, Leipzig, (1974).
3
Puech, J-L. PhD Thesis, University of Paul Sabatier, Tolouse (1978). [the lower level of cellulose is
probably unreliable]
4
Pettersen, R.C. In: ˇ®The Chemistry of Solid WoodˇŻ R. Rowell (ed.). pp. 57-127. Am. Chem. Soc.,
Washington D.C. (1984).
7](https://image.slidesharecdn.com/oakcompositionandmaturationinfluence-090727095255-phpapp02/85/Oak-Composition-And-Maturation-Influence-2-320.jpg)