Ofatumumab versus teriflunomide in multiple sclerosis
- 1. Ofatumumab versus Teriflunomide in Multiple Sclerosis S.L. Hauser, A. Bar-Or, J.A. Cohen, G. Comi, J. Correale, P.K. Coyle, A.H. Cross, J. de Seze, D. Leppert, X. Montalban, K. Selmaj, H. Wiendl, C. Kerloeguen, R. Willi, B. Li, A. Kakarieka, D. Tomic, A. Goodyear, R. Pingili, D.A. H├żring, K. Ramanathan, M. Merschhemke, and L. Kappos, for the ASCLEPIOS I and ASCLEPIOS II Trial Groups* e UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco, San Francisco (S.L.H.); the Center for Neuroinflammation and Experimental Therapeutics and Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia (A.B.-O.); the Department of Neurology, Mellen Center for Multiple Sclerosis, Neurological Institute, Cleveland Clinic, Cleveland (J.A.C.); the Institute of Experimental Neurology and Multiple Sclerosis Center IRCCS, San Raffaele Hospital, Milan (G.C.); the Department of Neurology, Fleni, Buenos Aires (J.C.); the Department of Neurology, Stony Brook University, Stony Brook, NY (P.K.C.); Washington University School of Medicine, St. Louis (A.H.C.); the University Hospital of Strasburg and Clinical Investigation Center INSERM 1434, Strasburg, France (J.S.); University Hospital Basel (D.L.), Novartis Pharma (C.K., R.W., A.K., D.T., D.A.H., K.R., M.M.), and the Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research, Biomedicine, and Biomedical Engineering, University Hospital and University of Basel (L.K.) ŌĆö all in Basel, Switzerland; the Department of NeurologyŌĆōNeuroimmunology, Centre dŌĆÖEsclerosi M├║ltiple de Catalunya (Cemcat), Hospital Universitari Vall dŌĆÖHebron, Barcelona (X.M.); the University of Warmia and Mazury, Olsztyn, and the Center of Neurology, Lodz ŌĆö both in Poland (K.S.); the Department of Neurology with Institute of Translational Neurology, University of M├╝nster, M├╝nster, Germany (H.W.); and Novartis Pharmaceuticals, East Hanover, NJ (B.L., A.G., R.P.). NEW ENGLAND JOURNAL OF MEDICINE AUGUST 2020
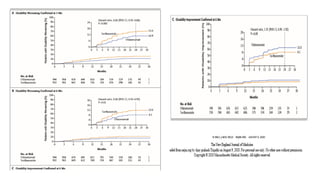
- 2. ŌĆó Ofatumumab, a subcutaneous anti-CD20 monoclonal antibody, selectively depletes B cells. Teriflunomide, an oral inhibitor of pyrimidine synthesis, reduces T-cell and B-cell activation. The relative effects of these two drugs in patients with multiple sclerosis are not known ŌĆó METHODS In two double-blind, double-dummy, phase 3 trials, we randomly assigned patients with relapsing multiple sclerosis to receive subcutaneous ofatumumab or oral teriflunomide ŌĆó Overall, 946 patients were assigned to receive ofatumumab and 936 to receive teriflunomide; the median follow-up was 1.6 year
- 5. DISCUSSION ŌĆó Annualised Relapse rate defined as the total number of relapses divided by the total person-day at risk of relapse multiplied by 365 ŌĆó In these two simultaneously conducted active controlled trials involving patients with relapsing multiple sclerosis, both ofatumumab and teriflunomide were associated with low relapse rates. The relapse rate was significantly lower with ofatumumab than with teriflunomide in each of the two trials ŌĆó Ofatumumab was also superior to teriflunomide in suppressing lesion activity on MRI ŌĆó Injection-related reactions were more frequent with ofatumumab