Organic chemistry Dr. Surendran Parambadath
Download as PPTX, PDF3 likes1,333 views
Dr. Surendran Parambadath is an Assistant Professor at Govt. Polytechnic College in Perinthalmanna, India. He was formerly a Post Doctoral Research Associate at Pusan National University in South Korea. The document provides information on the differences between organic and inorganic compounds, types of organic compounds including open chain, closed chain, aromatic and heterocyclic compounds. It also discusses properties like saturation, unsaturation and isomerism in organic chemistry.
1 of 27
Downloaded 63 times


























Recommended
Organic chemistry



Organic chemistryRavi Chandran
╠²
This document provides an overview of organic chemistry concepts, including:
1) Hydrocarbons are compounds made of only carbon and hydrogen, and can have single, double, or triple bonds between carbons.
2) Organic compounds derived from hydrocarbons include alcohols, ethers, aldehydes, ketones, acids, and esters which are formed by replacing hydrogen with other functional groups.
3) Macromolecules like proteins, carbohydrates, and nucleic acids are essential to life and control cellular activities and growth.Classification



ClassificationSandhyaPunetha1
╠²
This document provides an overview of the classification of organic compounds. It discusses the main categories of organic compounds including aliphatic (open-chain/saturated and unsaturated), cyclic (homocyclic and heterocyclic), and aromatic compounds. It describes the types of aliphatic, cyclic, and aromatic compounds in detail. It also introduces Huckel's rule for determining aromaticity based on the number of pi electrons in a conjugated cyclic system.Classification of organic compounds



Classification of organic compoundsAsaye Dessie
╠²
This document summarizes the classification of organic compounds. Organic compounds are broadly categorized into acyclic/open-chain compounds and cyclic/closed-chain compounds based on the arrangement of carbon atoms. Acyclic compounds have an open carbon chain that can be straight or branched. Cyclic compounds contain one or more closed rings and can be homocyclic containing only carbon, or heterocyclic containing other atoms. Homocyclic compounds are further divided into alicyclic and aromatic compounds. Aromatic compounds include benzenoid compounds containing benzene rings and non-benzenoid compounds containing other unsaturated rings.Organic molecules



Organic moleculesSiyavula
╠²
The document discusses organic chemistry and organic molecules. It defines organic molecules as those containing carbon and notes that all living things contain organic compounds. It describes the unique bonding properties of carbon that allow it to form many complex structures found in organic compounds. Finally, it discusses several classes of organic compounds like hydrocarbons, alcohols, carboxylic acids, and their properties and reactions.Classification of organic compounds



Classification of organic compoundsLahari Kumar
╠²
This document classifies organic compounds into two main categories: acyclic (open chain) compounds and cyclic (closed chain) compounds. Acyclic compounds contain open chains of carbon atoms, while cyclic compounds contain closed rings of carbon atoms. Within cyclic compounds, there are homocyclic compounds containing rings of only carbon atoms, and heterocyclic compounds containing rings with other atoms like nitrogen or oxygen in addition to carbon. Homocyclic compounds are further divided into alicyclic compounds resembling aliphatic properties and aromatic compounds containing alternating double and single bonds in rings.History, Classification, Uses of organic chemistry



History, Classification, Uses of organic chemistryAnm Sharif
╠²
Organic chemistry is the study of carbon-based compounds found in living things. The first organic chemist, Berzelius, believed organic compounds could only come from living organisms, but W├Čhler discovered the organic compound urea could be synthesized from inorganic precursors, disproving this idea of vitalism. Organic compounds make up the basic building blocks of life like carbohydrates, lipids, proteins, and nucleic acids and have a wide variety of uses from medicines to plastics.Organic molecules



Organic moleculesSiyavula
╠²
Organic chemistry deals with carbon-containing compounds called organic molecules. Carbon atoms can bond to many other atoms, often forming long chain structures. Organic compounds can be represented using molecular formulas or structural formulas. They contain functional groups that give them certain chemical properties and can be classified based on these groups. Organic reactions include addition, elimination, and substitution. Polymers are large molecules formed by combining repeating structural units (monomers) and include both natural and synthetic varieties.Organic macromolecules



Organic macromoleculesSiyavula
╠²
The document discusses organic macromolecules and polymers. It explains that polymers are made of monomers joined by covalent bonds, and can be natural or synthetic. The two main types of polymerization reactions are addition and condensation. The properties of polymers, such as strength and melting point, depend on the types of bonds between chains. Common synthetic polymers include plastics such as polystyrene, polyvinyl chloride, and polyethylene. Biological macromolecules like carbohydrates and proteins are also discussed.10.1 Introduction to Organic Chemistry



10.1 Introduction to Organic Chemistrydcroo1
╠²
The document discusses organic chemistry concepts including homologous series, isomers, and IUPAC nomenclature rules. It defines homologous series as groups of compounds with similar properties that differ by -CH2- units and a common generic formula. Isomers are described as compounds with the same molecular formula but different arrangements of atoms. The document provides examples of applying IUPAC nomenclature rules to name organic compounds containing up to six carbons and various functional groups.Organic chemistry review for Anatomy and Physiology Students



Organic chemistry review for Anatomy and Physiology StudentsJames H. Workman
╠²
Organic chemistry is the study of carbon-based compounds like those found in living things. Carbon can form diverse molecules through its ability to bond in many ways. Functional groups are parts of molecules that determine properties. The four main types of large biological molecules are carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates and lipids store energy, while proteins and nucleic acids have structural or information-storing roles like DNA. These molecules are made through polymerization of small repeating units into large complex structures essential for life.Carbon compounds (ppt)



Carbon compounds (ppt)Nabaneet Mondal
╠²
This ppt was made for our stupid projects..... The main purpose behind uploading this ppt is that no one should suffer like us and waste their time behind these stupid things... concentrate on your studies..Organic compound



Organic compoundAhura1
╠²
Organic compounds are almost 60% of all compounds. because of carbons tendency to form a compound as it has more than1 electron(4electrons) to form covallent compounds. SO a wide range of everything we eat is formed from carbon and hydrogen, which is the second important element to form organic compounds. Lesson 1 Organic Chemistry



Lesson 1 Organic ChemistryRichard Araneta
╠²
This document provides an introduction to organic chemistry. It discusses the history and development of organic chemistry as a science. The key points are:
1) Organic chemistry originated from the idea that organic materials possessed a "vital force" that distinguished them from inorganic materials.
2) Organic compounds are defined as compounds that contain carbon. Carbon atoms can form stable bonds to many other elements and a vast number of complex organic compounds.
3) The complexity of carbon-containing compounds allows for biologically important molecules like DNA, proteins, and carbohydrates. Organic chemistry is important for food, medicine, fuels and other industrial products.Short ppt onOrganic Chemistry



Short ppt onOrganic ChemistryAnkit Mahapatra
╠²
Organic chemistry is the study of carbon-containing compounds that are extremely important to life. Our bodies, medicines, fuels, plastics and many other materials are made of organic compounds. Carbon can form diverse compounds because it can form various bonds to itself and other elements. The structure and bonding of organic molecules determines their properties. Key groups like alcohols, ethers, carboxylic acids and esters impact a molecule's chemistry. There are several families of hydrocarbons including alkanes, alkenes and aromatics whose formulas vary based on saturation.Chapter 14 organic compounds



Chapter 14 organic compoundscoolscienceguy
╠²
Organic Chemistry
Hydrocarbons
Unsaturated Hydrocarbons
Functional Groups
Alcohols, Phenols, and Ethers
Amines and Alkaloids
Carbonyl Compounds
PolymersIntroduction to Organic Chemistry



Introduction to Organic Chemistryakshat saxena
╠²
A brief introduction to Organic Chemistry,its Origin, vital force theory, Breakdown of Vital force theory, categories of organic compounds.Organic chemistry



Organic chemistryobanbrahma
╠²
1) Organic chemistry is the study of carbon compounds and their properties. It is a separate discipline due to the vast number and variety of organic compounds, many of which are essential to life.
2) Carbon can form chains and rings by bonding to itself and other elements like hydrogen, oxygen, nitrogen and halogens. Functional groups like alcohols, aldehydes, ketones and carboxylic acids determine the properties and reactivity of organic molecules.
3) Chiral molecules are non-superimposable mirror images called enantiomers that can have different biological effects. The R/S system is used to distinguish these two forms.Advanced practical organic chemistry



Advanced practical organic chemistryAntonio Carlos Flash Batista
╠²
This document provides an introduction to organic chemistry. It discusses the key topics of functional groups, organic synthesis reagents, organic structure and reactions, elements involved in organic chemistry, and oxidation and reduction reactions. The main points are that organic chemistry is the study of carbon compounds and their properties and reactions, it is essential to life processes, and the carbon atom can form many different bonds giving rise to a huge variety of organic molecules and isomers.Chapter 20.1 : Introduction to Carbon and Organic Chemistry



Chapter 20.1 : Introduction to Carbon and Organic ChemistryChris Foltz
╠²
Carbon is the key element in organic chemistry due to its unique bonding properties. It can form single, double, and triple bonds with itself and other elements like hydrogen, oxygen, nitrogen, and sulfur. This allows for a huge diversity of organic compounds ranging from simple hydrocarbons like methane to more complex molecules. Organic chemists use structural formulas to represent the arrangement and bonding of atoms within organic molecules, which is important for determining properties. Isomers are compounds with the same molecular formula but different structures or arrangements of atoms.Organic Compounds



Organic CompoundsJenny Dixon
╠²
This document provides information about organic compounds and their components. It defines organic compounds as those containing carbon bonded to itself, hydrogen, and other elements like oxygen, nitrogen, phosphorus or sulfur. Examples of organic compounds that make up living things are described, including carbohydrates, lipids, proteins, and nucleic acids. These compounds are composed of combinations of the elements carbon, hydrogen, oxygen, nitrogen, phosphorus and sometimes sulfur. The document emphasizes carbon's unique ability to form many diverse molecules by bonding to itself and other elements.Intro to Organic chemistry



Intro to Organic chemistryJennyfer Merabe
╠²
Organic Chemistry
1. History
2. Properties of Organic Chemistry
3. comparison of Compounds
4. Sources of Organic Compounds
5. Types of Organic Compounds
6.Types of Organic Formula
7. Carbon
8. Structural Formulas of Carbon
9. Isomerism
10 Classification of Organic Compounds
11. HydroCarbonsOrganic chemistry-basic concepts-1



Organic chemistry-basic concepts-1janardhan acharyulu
╠²
Organic compounds are vital for life and include substances like DNA, proteins, fuels, polymers, dyes, and medicines. Carbon has the unique ability to form covalent bonds with itself and other elements through a process called catenation. The shapes of organic molecules like methane, ethene, and ethyne are determined by the hybridization of carbon - methane is tetrahedral due to sp3 hybridization, ethene is planar due to sp2 hybridization, and ethyne is linear due to sp hybridization. Hybridization also influences bond lengths and energies. ŽĆ-bonds form through parallel sideways overlap of p-orbitals on adjacent carbons and have electron density above and below the plane of the bonded atomsPoc 1 1 classification, nomenclature and isomerism of organic compounds



Poc 1 1 classification, nomenclature and isomerism of organic compoundsomkar ghatge
╠²
The document discusses the classification, nomenclature, and structural isomerism of organic compounds. It describes how organic compounds can be classified as acyclic, alicyclic, aromatic, and heterocyclic. The IUPAC system of nomenclature is also outlined, including rules for naming parent chains, substituents, and complex groups. Finally, different types of structural isomerism are defined, such as chain, position, functional, metamerism, tautomerism, and ring-chain isomerism.Notes



NotesBruce Coulter
╠²
This document provides an overview of organic chemistry, including key topics such as:
- Organic compounds contain carbon and are found in many common materials.
- Organic chemistry is the study of organic compounds, their structures, properties, and reactions.
- Carbon atoms can form multiple bonds with other carbons, allowing for a large number of organic compounds.
- Hydrocarbons are organic compounds made of only carbon and hydrogen, and can be classified as aliphatic or aromatic.Organic compound ppt



Organic compound pptKanishkNegi5
╠²
Organic chemistry is the study of carbon compounds, both those produced by living organisms and those produced synthetically. It includes the structure, properties, reactions and preparation of carbon-based molecules. Organic compounds can be classified as natural or synthetic, and include substances such as sugars, carbohydrates, proteins, polymers and hydrocarbons of different lengths formed from carbon and hydrogen atoms. Decomposition is the breakdown of dead organic matter through biological and biochemical processes that release inorganic nutrients.Introduction of organic chemistry



Introduction of organic chemistrySaiful Najmi
╠²
Organic chemistry is the study of carbon compounds. The document introduces organic chemistry, discussing the history and key figures in the field. It describes the properties of carbon that allow it to form many different compounds and categorizes the main types and sources of organic compounds, including naturally occurring, synthetic, and invented compounds. Organic compounds have applications in areas like medicine, pesticides, dyes, plastics, and more.Unit 16 Carbon Chemistry



Unit 16 Carbon ChemistryOlympus High School - Jeff Taylor
╠²
The document discusses organic chemistry concepts, including:
- Organic compounds contain carbon and are found in living and once-living organisms. Carbon can form up to four covalent bonds, allowing for complex molecules.
- Hydrocarbons are the simplest organic compounds, made of only carbon and hydrogen. They have properties like low melting/boiling points and flammability.
- Hydrocarbons can be saturated or unsaturated. Saturated hydrocarbons have the maximum number of hydrogen atoms, while unsaturated ones have double or triple bonds.
- Structural formulas show how atoms are arranged in molecules, important for understanding isomers that have the same molecular formula but different structures.Unit 4ale



Unit 4alealekey08
╠²
This document provides an introduction to organic chemistry, including definitions of key terms and concepts. It discusses:
- The early history of organic chemistry and the discovery that organic compounds could be synthesized in the lab.
- The main differences between organic and inorganic compounds in terms of their properties and bonding.
- The central role of carbon atoms in organic compounds and their ability to form chains and complex structures through catenation.
- The different classes of hydrocarbons including alkanes, alkenes, alkynes, aromatics, and their IUPAC naming conventions.
- Important organic functional groups derived from hydrocarbons like alkyl halides, alcohols, ethers, alUnit 4



Unit 4alekey08
╠²
This document provides an introduction to organic chemistry, including definitions of organic compounds, differences between organic and inorganic compounds, and key concepts. It discusses the early history when vitalism prevented the synthesis of organic compounds. Friedrich W├Čhler was the first to synthesize an organic compound in a laboratory. The document also outlines types of organic compounds like hydrocarbons, and how they are named according to IUPAC rules. Carbon properties and different hybridizations that allow multiple bonds are covered.Carbon and its compounds



Carbon and its compoundsJay Parekh
╠²
* CARBON is the chemical element with symbol C and atomic number 6. As a member of group IV on the periodic table, it is nonmetallic and tetravalentŌĆömaking four electrons available to form covalent chemical bonds.
* Bonding in Carbon-Covalent Bond
* Allotropes of Carbon
* Graphite
* Diamond
* Fullerenes
* Organic Chemistry
* Isomerism
* SoapsMore Related Content
What's hot (19)
10.1 Introduction to Organic Chemistry



10.1 Introduction to Organic Chemistrydcroo1
╠²
The document discusses organic chemistry concepts including homologous series, isomers, and IUPAC nomenclature rules. It defines homologous series as groups of compounds with similar properties that differ by -CH2- units and a common generic formula. Isomers are described as compounds with the same molecular formula but different arrangements of atoms. The document provides examples of applying IUPAC nomenclature rules to name organic compounds containing up to six carbons and various functional groups.Organic chemistry review for Anatomy and Physiology Students



Organic chemistry review for Anatomy and Physiology StudentsJames H. Workman
╠²
Organic chemistry is the study of carbon-based compounds like those found in living things. Carbon can form diverse molecules through its ability to bond in many ways. Functional groups are parts of molecules that determine properties. The four main types of large biological molecules are carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates and lipids store energy, while proteins and nucleic acids have structural or information-storing roles like DNA. These molecules are made through polymerization of small repeating units into large complex structures essential for life.Carbon compounds (ppt)



Carbon compounds (ppt)Nabaneet Mondal
╠²
This ppt was made for our stupid projects..... The main purpose behind uploading this ppt is that no one should suffer like us and waste their time behind these stupid things... concentrate on your studies..Organic compound



Organic compoundAhura1
╠²
Organic compounds are almost 60% of all compounds. because of carbons tendency to form a compound as it has more than1 electron(4electrons) to form covallent compounds. SO a wide range of everything we eat is formed from carbon and hydrogen, which is the second important element to form organic compounds. Lesson 1 Organic Chemistry



Lesson 1 Organic ChemistryRichard Araneta
╠²
This document provides an introduction to organic chemistry. It discusses the history and development of organic chemistry as a science. The key points are:
1) Organic chemistry originated from the idea that organic materials possessed a "vital force" that distinguished them from inorganic materials.
2) Organic compounds are defined as compounds that contain carbon. Carbon atoms can form stable bonds to many other elements and a vast number of complex organic compounds.
3) The complexity of carbon-containing compounds allows for biologically important molecules like DNA, proteins, and carbohydrates. Organic chemistry is important for food, medicine, fuels and other industrial products.Short ppt onOrganic Chemistry



Short ppt onOrganic ChemistryAnkit Mahapatra
╠²
Organic chemistry is the study of carbon-containing compounds that are extremely important to life. Our bodies, medicines, fuels, plastics and many other materials are made of organic compounds. Carbon can form diverse compounds because it can form various bonds to itself and other elements. The structure and bonding of organic molecules determines their properties. Key groups like alcohols, ethers, carboxylic acids and esters impact a molecule's chemistry. There are several families of hydrocarbons including alkanes, alkenes and aromatics whose formulas vary based on saturation.Chapter 14 organic compounds



Chapter 14 organic compoundscoolscienceguy
╠²
Organic Chemistry
Hydrocarbons
Unsaturated Hydrocarbons
Functional Groups
Alcohols, Phenols, and Ethers
Amines and Alkaloids
Carbonyl Compounds
PolymersIntroduction to Organic Chemistry



Introduction to Organic Chemistryakshat saxena
╠²
A brief introduction to Organic Chemistry,its Origin, vital force theory, Breakdown of Vital force theory, categories of organic compounds.Organic chemistry



Organic chemistryobanbrahma
╠²
1) Organic chemistry is the study of carbon compounds and their properties. It is a separate discipline due to the vast number and variety of organic compounds, many of which are essential to life.
2) Carbon can form chains and rings by bonding to itself and other elements like hydrogen, oxygen, nitrogen and halogens. Functional groups like alcohols, aldehydes, ketones and carboxylic acids determine the properties and reactivity of organic molecules.
3) Chiral molecules are non-superimposable mirror images called enantiomers that can have different biological effects. The R/S system is used to distinguish these two forms.Advanced practical organic chemistry



Advanced practical organic chemistryAntonio Carlos Flash Batista
╠²
This document provides an introduction to organic chemistry. It discusses the key topics of functional groups, organic synthesis reagents, organic structure and reactions, elements involved in organic chemistry, and oxidation and reduction reactions. The main points are that organic chemistry is the study of carbon compounds and their properties and reactions, it is essential to life processes, and the carbon atom can form many different bonds giving rise to a huge variety of organic molecules and isomers.Chapter 20.1 : Introduction to Carbon and Organic Chemistry



Chapter 20.1 : Introduction to Carbon and Organic ChemistryChris Foltz
╠²
Carbon is the key element in organic chemistry due to its unique bonding properties. It can form single, double, and triple bonds with itself and other elements like hydrogen, oxygen, nitrogen, and sulfur. This allows for a huge diversity of organic compounds ranging from simple hydrocarbons like methane to more complex molecules. Organic chemists use structural formulas to represent the arrangement and bonding of atoms within organic molecules, which is important for determining properties. Isomers are compounds with the same molecular formula but different structures or arrangements of atoms.Organic Compounds



Organic CompoundsJenny Dixon
╠²
This document provides information about organic compounds and their components. It defines organic compounds as those containing carbon bonded to itself, hydrogen, and other elements like oxygen, nitrogen, phosphorus or sulfur. Examples of organic compounds that make up living things are described, including carbohydrates, lipids, proteins, and nucleic acids. These compounds are composed of combinations of the elements carbon, hydrogen, oxygen, nitrogen, phosphorus and sometimes sulfur. The document emphasizes carbon's unique ability to form many diverse molecules by bonding to itself and other elements.Intro to Organic chemistry



Intro to Organic chemistryJennyfer Merabe
╠²
Organic Chemistry
1. History
2. Properties of Organic Chemistry
3. comparison of Compounds
4. Sources of Organic Compounds
5. Types of Organic Compounds
6.Types of Organic Formula
7. Carbon
8. Structural Formulas of Carbon
9. Isomerism
10 Classification of Organic Compounds
11. HydroCarbonsOrganic chemistry-basic concepts-1



Organic chemistry-basic concepts-1janardhan acharyulu
╠²
Organic compounds are vital for life and include substances like DNA, proteins, fuels, polymers, dyes, and medicines. Carbon has the unique ability to form covalent bonds with itself and other elements through a process called catenation. The shapes of organic molecules like methane, ethene, and ethyne are determined by the hybridization of carbon - methane is tetrahedral due to sp3 hybridization, ethene is planar due to sp2 hybridization, and ethyne is linear due to sp hybridization. Hybridization also influences bond lengths and energies. ŽĆ-bonds form through parallel sideways overlap of p-orbitals on adjacent carbons and have electron density above and below the plane of the bonded atomsPoc 1 1 classification, nomenclature and isomerism of organic compounds



Poc 1 1 classification, nomenclature and isomerism of organic compoundsomkar ghatge
╠²
The document discusses the classification, nomenclature, and structural isomerism of organic compounds. It describes how organic compounds can be classified as acyclic, alicyclic, aromatic, and heterocyclic. The IUPAC system of nomenclature is also outlined, including rules for naming parent chains, substituents, and complex groups. Finally, different types of structural isomerism are defined, such as chain, position, functional, metamerism, tautomerism, and ring-chain isomerism.Notes



NotesBruce Coulter
╠²
This document provides an overview of organic chemistry, including key topics such as:
- Organic compounds contain carbon and are found in many common materials.
- Organic chemistry is the study of organic compounds, their structures, properties, and reactions.
- Carbon atoms can form multiple bonds with other carbons, allowing for a large number of organic compounds.
- Hydrocarbons are organic compounds made of only carbon and hydrogen, and can be classified as aliphatic or aromatic.Organic compound ppt



Organic compound pptKanishkNegi5
╠²
Organic chemistry is the study of carbon compounds, both those produced by living organisms and those produced synthetically. It includes the structure, properties, reactions and preparation of carbon-based molecules. Organic compounds can be classified as natural or synthetic, and include substances such as sugars, carbohydrates, proteins, polymers and hydrocarbons of different lengths formed from carbon and hydrogen atoms. Decomposition is the breakdown of dead organic matter through biological and biochemical processes that release inorganic nutrients.Introduction of organic chemistry



Introduction of organic chemistrySaiful Najmi
╠²
Organic chemistry is the study of carbon compounds. The document introduces organic chemistry, discussing the history and key figures in the field. It describes the properties of carbon that allow it to form many different compounds and categorizes the main types and sources of organic compounds, including naturally occurring, synthetic, and invented compounds. Organic compounds have applications in areas like medicine, pesticides, dyes, plastics, and more.Unit 16 Carbon Chemistry



Unit 16 Carbon ChemistryOlympus High School - Jeff Taylor
╠²
The document discusses organic chemistry concepts, including:
- Organic compounds contain carbon and are found in living and once-living organisms. Carbon can form up to four covalent bonds, allowing for complex molecules.
- Hydrocarbons are the simplest organic compounds, made of only carbon and hydrogen. They have properties like low melting/boiling points and flammability.
- Hydrocarbons can be saturated or unsaturated. Saturated hydrocarbons have the maximum number of hydrogen atoms, while unsaturated ones have double or triple bonds.
- Structural formulas show how atoms are arranged in molecules, important for understanding isomers that have the same molecular formula but different structures.Similar to Organic chemistry Dr. Surendran Parambadath (20)
Unit 4ale



Unit 4alealekey08
╠²
This document provides an introduction to organic chemistry, including definitions of key terms and concepts. It discusses:
- The early history of organic chemistry and the discovery that organic compounds could be synthesized in the lab.
- The main differences between organic and inorganic compounds in terms of their properties and bonding.
- The central role of carbon atoms in organic compounds and their ability to form chains and complex structures through catenation.
- The different classes of hydrocarbons including alkanes, alkenes, alkynes, aromatics, and their IUPAC naming conventions.
- Important organic functional groups derived from hydrocarbons like alkyl halides, alcohols, ethers, alUnit 4



Unit 4alekey08
╠²
This document provides an introduction to organic chemistry, including definitions of organic compounds, differences between organic and inorganic compounds, and key concepts. It discusses the early history when vitalism prevented the synthesis of organic compounds. Friedrich W├Čhler was the first to synthesize an organic compound in a laboratory. The document also outlines types of organic compounds like hydrocarbons, and how they are named according to IUPAC rules. Carbon properties and different hybridizations that allow multiple bonds are covered.Carbon and its compounds



Carbon and its compoundsJay Parekh
╠²
* CARBON is the chemical element with symbol C and atomic number 6. As a member of group IV on the periodic table, it is nonmetallic and tetravalentŌĆömaking four electrons available to form covalent chemical bonds.
* Bonding in Carbon-Covalent Bond
* Allotropes of Carbon
* Graphite
* Diamond
* Fullerenes
* Organic Chemistry
* Isomerism
* SoapsOrganic Chemistry Introduction and Orbital Design



Organic Chemistry Introduction and Orbital DesignPharmacy Universe
╠²
Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.P O S T L A B Biochem D L S L



P O S T L A B Biochem D L S LKim Harold Caponpon
╠²
This document summarizes key differences between organic and inorganic compounds based on a biochemistry lab experiment. Organic compounds contain carbon and are found in living organisms, while inorganic compounds do not contain carbon or result from non-living processes. The document outlines tests to detect carbon, hydrogen, oxygen, nitrogen, and sulfur in organic compounds, such as evolving gases upon heating with bases or forming precipitates with reagents. Common organic compounds and their properties are also discussed.Organic Chemistry: Introductory Topics



Organic Chemistry: Introductory Topicswewwchemistry
╠²
[ Visit http://www.wewwchemistry.com ] This is a summary presentation of the introductory topics in Organic Chemistry, prepared according to the Singapore-Cambridge GCE A Level 9647 H2 Chemistry syllabus.Org chem introduction (seu)



Org chem introduction (seu)asraf sohel
╠²
Organic compounds can be classified based on their functional groups. Members of the same homologous series have similar chemical properties and their physical properties change gradually with increasing carbon chain length. Key factors that affect the physical properties of organic compounds include the structure of the functional group, length of carbon chains, and ability to form hydrogen bonds or dipole-dipole interactions. Non-polar compounds have lower boiling points than polar compounds due to weaker intermolecular forces.Introduction to organic chemistry Foundation In science



Introduction to organic chemistry Foundation In scienceMSU MALAYSIA
╠²
Organic chemistry is the study of carbon compounds. The four main types of hydrocarbons are saturated, unsaturated, aliphatic, and aromatic. Saturated hydrocarbons contain only single bonds and their general formula is CnH2n+2. Unsaturated hydrocarbons contain double or triple bonds and include alkenes and alkynes. Aromatic hydrocarbons contain benzene or benzene-like rings. Functional groups are atoms or groups of atoms that are largely responsible for the chemical behavior of organic compounds. Common functional groups include alcohols, aldehydes, ketones, carboxylic acids, esters, and amines. Organic compounds can exhibit isomerism when theyOrganic-Chemistry Introduction Chembiooo



Organic-Chemistry Introduction Chembiooormayukilchmsu
╠²
This document provides an introduction to organic chemistry. It discusses that organic chemistry is the study of carbon compounds and their structures and reactions. Over 16 million carbon compounds are known. Carbon can form stable chains and rings due to its strong single and double bonds. Functional groups are atoms or groups that are involved in characteristic chemical reactions. Hydrocarbons only contain carbon and hydrogen, and homologous series differ by CH2 units. Isomers have the same molecular formula but different structures. The document also discusses alkenes, alkynes and alkyl halides.Introduction To Carbon Compound



Introduction To Carbon CompoundMohd Norihwan
╠²
This document provides a summary of Lesson 1 from an organic chemistry textbook chapter on carbon compounds. It covers 10 learning objectives related to the unique properties of carbon, isomers, functional groups, saturated vs unsaturated compounds, and IUPAC naming conventions. Key topics include how carbon can form multiple bonds and chains/rings, the importance of functional groups for classifying compounds, and systematic naming of organic molecules.Introduction to organic chemistry



Introduction to organic chemistryjagan vana
╠²
Carbon forms a huge number of compounds due to its ability to form strong bonds with itself and other elements. The study of carbon-based compounds is called organic chemistry. Organic compounds are classified based on their carbon skeleton and functional groups. Key aspects of organic chemistry include structural and stereoisomers, functional groups that determine reactivity, and IUPAC nomenclature for systematic naming.Carbon and Its Compound



Carbon and Its CompoundDr. Pranabjyoti Das
╠²
Carbon is a versatile element that forms millions of compounds. It exists in many forms including diamond and graphite. Carbon is present in all living organisms and is the main component of fuels like coal.
Carbon atoms bond with other atoms through covalent bonds by sharing electrons. This allows carbon to form chains, branches and closed rings. Hydrocarbons contain only carbon and hydrogen and can be saturated or unsaturated. Functional groups determine the properties of carbon compounds.
Some important carbon compounds are ethanol, ethanoic acid, and soaps. Ethanol is used in drinks and medicines while ethanoic acid gives vinegar its sour taste. Soaps clean through micelle formation while detergents work better inorganic.doc



organic.docReneeRamdial3
╠²
Organic chemistry is the study of carbon compounds. Carbon forms strong covalent bonds and can form long chains and rings, resulting in a vast number of possible structures. Organic molecules are classified based on their functional groups, such as alkanes (no functional group), alkenes (C=C double bond), and haloalkanes (halogen atom attached to carbon). Isomers are compounds with the same molecular formula but different structures, including positional isomers (functional group in a different position), chain isomers (different carbon skeleton arrangement), and functional isomers (different functional groups). Nomenclature involves naming compounds based on the parent chain, functional groups, and location of any branches.bonding in carbon compounds



bonding in carbon compoundsMonique Anderson
╠²
This document discusses carbon bonding and the formation of carbon compounds. It explains that carbon can form strong covalent bonds with other carbon atoms through a process called catenation, allowing it to form straight chains, branches, and rings. This bonding ability arises because carbon is tetravalent and can hybridize its orbitals, taking on different hybridization states like sp, sp2, and sp3. Some carbon compounds exhibit resonance, where electrons are delocalized over multiple carbon atoms. This results in more stable structures that are hybrids of different resonant forms. Overall, carbon's unique bonding properties allow it to form a diverse array of stable organic compounds.Biochemistry for nursing Chemistry of Life.pptx



Biochemistry for nursing Chemistry of Life.pptxGabrielVillegas37
╠²
The document discusses the key differences between organic and inorganic compounds. Organic compounds contain carbon and have properties like being flammable, having lower melting and boiling points, forming covalent bonds, and containing complex molecular structures. The document outlines the major classes of organic compounds including aliphatic, aromatic, heterocyclic, and alicyclic compounds. It also provides examples of hydrocarbon compounds like alkanes, alkenes, and alkynes as well as functional groups including alcohols, aldehydes, ketones, ethers, carboxylic acids, esters, and alkyl halides. Amines are also listed as an important class of organic compounds.CARBON AND ITS COMPUNDS.pptx



CARBON AND ITS COMPUNDS.pptxDeepika195894
╠²
Carbon is a nonmetal that is the fourth most abundant element in the human body and is essential to life. It has several allotropes including diamond, graphite, and buckminsterfullerene. Carbon forms covalent bonds and can bond to other carbon atoms to form chains, rings, and complex molecules. Many organic compounds are based on carbon chains and rings that may be saturated or unsaturated. Carbon undergoes many important reactions including combustion, oxidation, and substitution reactions.Lecturenote10 13



Lecturenote10 13dustinjohn
╠²
This document provides an overview of organic chemistry concepts including alkanes, alkenes, alkynes, and benzene. It discusses key topics such as:
- Organic compounds contain carbon and may also contain hydrogen, oxygen, nitrogen, and other elements.
- There are two main ways organic compounds are obtained: isolation from nature and synthesis in the laboratory.
- Hydrocarbons are divided into saturated (alkanes) and unsaturated (alkenes and alkynes) groups based on the presence of double or triple carbon bonds.
- Nomenclature rules are provided for naming straight-chain, branched, cyclic, and substituted organic compounds according to IUPAC conventions. Or Ganic Intro



Or Ganic Introscuffruff
╠²
This document provides an introduction to organic chemistry for A-level students. It begins with an overview of organic chemistry and the special properties of carbon that allow for the vast number of carbon compounds. It then discusses specific topics like functional groups, isomers, naming conventions (IUPAC), and more. The document is intended to help students understand key concepts in organic chemistry.More from Surendran Parambadath (11)
Soaps and detergents Dr. surendran parambadath



Soaps and detergents Dr. surendran parambadathSurendran Parambadath
╠²
Dr. Surendran Parambadath is an Assistant Professor of Chemistry at TM Jacob Memorial Govt. College in Manimalakunnu. The document defines soaps as sodium or potassium salts of higher fatty acids like stearic, palmitic, and oleic acids. It discusses the types of soaps including hard soaps, soft soaps, transparent soaps, baby soaps, and liquid soaps. The document also covers total fatty matter, grades of soap, bathing bars, and the cleansing action of soap.Nanomaterials dr.surendran prambadath



Nanomaterials dr.surendran prambadathSurendran Parambadath
╠²
This document provides an overview of nanomaterials and carbon nanotubes. It discusses how nanomaterials are materials with sizes between 1 to 100 nm that exhibit unique properties. Carbon nanotubes are nanomaterials made of rolled graphene sheets that have excellent mechanical and electrical properties. The document outlines several methods for synthesizing carbon nanotubes including high pressure carbon monoxide deposition and chemical vapor deposition. It then discusses important properties and applications of carbon nanotubes such as their strength, conductivity, and use as reinforcements in composites.Formula and equations dr.surendran prambadath



Formula and equations dr.surendran prambadathSurendran Parambadath
╠²
The document discusses various topics in science including chemistry, physics, biology, geology, polymer science, medicine, materials science, and nanotechnology. It then provides information about atoms, molecules, inorganic and organic chemistry, physical and analytical chemistry.Acids and bases dr.surendran prambadath



Acids and bases dr.surendran prambadathSurendran Parambadath
╠²
The document discusses acids and bases according to different theories including Arrhenius, Bronsted-Lowry, and Lewis concepts. It defines acids and bases, describes their properties, and explains neutralization reactions. Examples are provided of strong vs weak acids and bases as well as monoprotic, diprotic, and triprotic acids and bases based on their equivalent weights.P h dr.surendran prambadath



P h dr.surendran prambadathSurendran Parambadath
╠²
The document discusses pH and the ionization of water. It defines pH as the negative logarithm of the hydrogen ion concentration and explains that pH values are used to determine if a solution is acidic, basic, or neutral. A pH of 7 is neutral, below 7 is acidic, and above 7 is basic. Buffer solutions are also introduced, which help maintain a stable pH when small amounts of acid or base are added. Key applications of measuring pH are mentioned, such as in corrosion, water treatment, soil chemistry, and medicine.Surface chemistry-Dr. Surendran Parambadath



Surface chemistry-Dr. Surendran ParambadathSurendran Parambadath
╠²
The document discusses adsorption, which is the accumulation of molecules on the surface of solids or liquids. It defines key terms like adsorbate, adsorbent, desorption, and occlusion. The document also distinguishes between physisorption and chemisorption, and notes factors that influence adsorption like surface area, temperature, and pressure. Some applications of adsorption are mentioned as well, such as in gas masks, vacuum production, water softening, catalysis, petroleum refining, and chromatography.Polumers-Dr. Surendran Parambadath



Polumers-Dr. Surendran ParambadathSurendran Parambadath
╠²
1. The document discusses polymers, their types including plastics, elastomers, fibers, and composites.
2. Key polymerization reactions are addition polymerization which does not eliminate small molecules, and condensation polymerization which eliminates molecules like water.
3. Common polymers discussed include nylon, polyethylene, polyester, rubber and fibers like nylon-6,6 and terylene. Composites contain a matrix and dispersed strengthening phase.Fuels-Dr. Surendran Parambadath



Fuels-Dr. Surendran ParambadathSurendran Parambadath
╠²
This document provides information on various types of fuels including their classification, composition, and uses. It discusses natural/primary fuels like wood, coal, petroleum and natural gas. It also describes artificial/secondary fuels produced from primary fuels such as coke, kerosene, petrol and diesel. Liquid fuels mainly come from petroleum and its distillation fractions. Gaseous fuels discussed include natural gas, water gas, producer gas, biogas, LPG and CNG. The characteristics of good fuels and fuel values are also summarized.Environmental chemistry-Dr. Surendran Parambadath



Environmental chemistry-Dr. Surendran ParambadathSurendran Parambadath
╠²
This document provides an overview of various types of environmental pollution including air, water, soil, noise, thermal, and radioactive pollution. It discusses the causes and effects of each type of pollution and potential methods for prevention and control. The key points covered are:
1. It defines pollution and categorizes it into six main types: air, water, soil, noise, thermal, and radioactive pollution.
2. For each type of pollution, it discusses sources, harmful effects, and potential approaches to prevention and control such as reducing contaminants at the source, modifying processes, and proper treatment and disposal.
3. It emphasizes the need to control pollution through approaches like proper industrial siting, waste treatment, affCorrosion-Dr. Surendran Parambadath



Corrosion-Dr. Surendran ParambadathSurendran Parambadath
╠²
This document discusses corrosion and how it affects metals. It defines corrosion as the slow process of decay of metals due to chemical or electrochemical reaction with their environment. This causes the formation of compounds like oxides on the metal surface. Corrosion occurs through both dry chemical reactions and wet electrochemical reactions that set up galvanic cells. The rate of corrosion is affected by factors like the metal's position in the galvanic series, purity, environment, and characteristics of the corrosion products formed. Common types of corrosion discussed are rusting of iron and methods to prevent or reduce corrosion like coatings, cathodic protection, and modifying the environment.Electrochemistry-Dr. Surendran Parambadath



Electrochemistry-Dr. Surendran ParambadathSurendran Parambadath
╠²
The document discusses different types of electrochemical cells including primary cells that produce electricity from non-reversible chemical reactions and secondary cells that can be recharged by passing electricity in the opposite direction of the spontaneous reaction. Examples of primary cells discussed include Daniel, mercury, dry, and alkaline cells, while examples of secondary cells include lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries. The working and reactions of common cells like lead-acid, alkaline, and dry cells are also explained.Organic chemistry Dr. Surendran Parambadath
- 2. Dr. SURENDRAN PARAMBADATH (M.Sc, M.Phil, M.Tech) Formerly: Post Doctoral Research Associate, Nano-Information Materials Research Laboratory, Pusan National University, Busan-South Korea Currently: Assistant Professor Govt. Polytechnic College, Perinthalmanna
- 4. Organic Compound Inorganic Compound 1 Found in living organisms Found in non living matters-minerals 2 Besides carbon they are compounds of H, They are composed of one or more of any O, N, S and P of the known elements. 3 They are covalent compounds They are electrovalent compounds 4 They are volatile and inflammable They are generally nonvolatile and non inflammable 5 Generally insoluble in water and soluble in Soluble in water and insoluble in non polar non polar solvents, like benzene. solvents. 6 Reaction involving organic compounds are Reaction is fast slow 7 They exhibit isomerism Only coordination compounds exhibit isomerism 8 They are non conductors of electricity Many of them conduct electricity in solution or fused form 9 Their number is very large Small in number 10 Generally, solids, liquids or gases Generally liquids or high melting solids.
- 5. The number of organic compounds is very large (~90%) Because: 1. Catenation capacity 2. Strength of C-C bond 3. Tetra-covalency 4. Capacity to combine with other non-metals like, H, O, N, S, P, Cl, Br, I etc. 5. Possibility of multiple bonds and 6. Isomerism
- 6. The tendency of an element to form chain of identical atoms is called catenation. Due to catenation, carbon atoms can form straight chains, branched chains or closed chain compounds. C C C C C C C C C C C C C C C C C C C C C C C C C C C C C C C
- 8. Compared to Si-Si, S-S, N-N or O-O bond C-C bond is stronger and requires higher energy for breaking. Hence chains with C-C bonds are stable.
- 9. Carbon atoms has four electrons in its valence shell and can form four covalent bonds. This tetra-covalency or quadri covalency of carbon gives rise to various possibilities for formation of variety of structures. C C C C
- 10. Isomerism is the phenomenon in which one and the same molecular formula represents more than one compound with different properties. Due to directional character of covalent bonds different spacial arrangements and isomers are possible for compounds with the same structural formula. CH3 H3C CH3 CH3 Trans-2-butene cis-2-butene
- 12. Organic compounds with only single covalent bonds between the carbon atoms are called saturated compounds. CH3-CH2-CH2-CH2-CH3 In saturated compounds there will be no double or triple bond between carbon atoms. In unsaturated compound, double or triple bond between carbon atoms. CH3-CH=CH-CH2-CH3
- 13. 1. Unsaturated compounds decolorizes yellow colored bromine water. 2. Baeyrs test: Unsaturated compounds decolorize alkaline potassium permanganate solution. Saturated Unsaturated Contain only single covalent bonds Contain at least one covalent between carbon atoms. double or triple bond between carbon atoms. Less reactive More reactive Does not decolorize bromine water Decolorize bromine water
- 14. Open Chain (Acyclic or aliphatic) Compounds These compounds contain an open chain of carbon atoms which may be a straight or a branched chain. CH3-CH2-CH2-CH3 CH3 Normal butane (n-butane) Straight chain CH3-CH-CH3 Isobutane (iso-butane) Branched chain
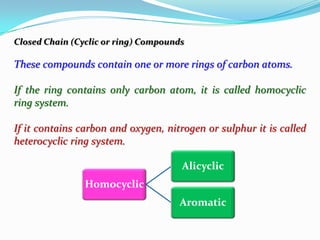
- 15. Closed Chain (Cyclic or ring) Compounds These compounds contain one or more rings of carbon atoms. If the ring contains only carbon atom, it is called homocyclic ring system. If it contains carbon and oxygen, nitrogen or sulphur it is called heterocyclic ring system. Alicyclic Homocyclic Aromatic
- 16. Cyclopropane Cyclobutane Benzene N O O Oxazole Furan Naphthalene
- 17. Organic Compound Closed Open Chain Chain Straight Branched Heterocyclic Homocyclic Chain Chain Alicyclic Aromatic
- 19. Alicyclic
- 20. Heterocyclic
- 22. Class of Nature of the Formula of the Example Organic functional functional Compound group group Alkenes Double bond C=C Ethene Alkynes Triple bond -C’é║C- Acetylene Alcohols Hydroxy -O-H Methanol Aldehydes Aldehydic -CHO Acetaldehyde Group Acids Carboxyl -COOH Acetic acid Ethers Ether group -O- Diethyl ether Ketones Ketonic group -CO- Acetone Amines Amino group -NH2 Methyl amine Esters Ester group -COO- Methyl acetate
- 23. Isomerism Two or more compounds having the same molecular formula but different properties are called isomers and the phenomenon is called isomerism. Structural: If the difference in properties of two isomers is due to difference in their structure, it is called structural isomerism. Stereo: If the isomers have the same structure but different configuration it is called stereoisomerism.
- 24. Structural isomerism CH3-CH2-CH2-CH3 CH3 Normal butane (n-butane) CH3-CH-CH3 Isobutane (iso-butane) Molecular Formula: C4H10
- 25. CH3-CH2-CH2-OH OH Normal propyl alcohol CH3-CH-CH3 Isopropyl alcohol Molecular Formula: C3H7-OH
- 26. Stereo isomerism CH3 H3C CH3 CH3 Trans-2-butene cis-2-butene


