Overview of Quality Management System for AEFI Surveillance training ppt_V1.pptx
- 1. National Quality Assurance Standards for AEFI surveillance Presented by Dr Ankur Raghav National AEFI Secretariat Ministry of Health and Family Welfare Government of India
- 2. NQAS for AEFI Surveillance System ? Recommended by WHO’s assessment of the National Regulatory Authority (NRA) in 2012 ? Set up and implement a Quality Management System for AEFI surveillance processes across the country ? Objective - to improve the quality and efficiency of AEFI surveillance processes at all levels ? Trainings and capacity building, documentation and monitoring of QMS implementation play key roles in quality assurance. ? Key activities, focus areas, indicators and benchmarks are identified and defined ? Standards for quality assurance in AEFI surveillance ? Based on the National Quality Assurance System for Public Health Facilities ? Supported by the Quality Improvement Division of National Health Systems Resource Center (NHSRC), New Delhi ? Requires coordination between immunization team and quality assurance team at state and district level
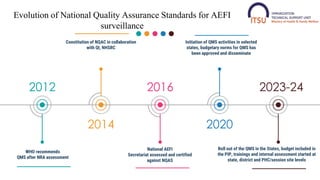
- 3. 2012 WHO recommends QMS after NRA assessment 2014 Constitution of NQAC in collaboration with QI, NHSRC 2016 National AEFI Secretariat assessed and certified against NQAS 2020 Initiation of QMS activities in selected states, budgetary norms for QMS has been approved and disseminate 2023-24 Evolution of National Quality Assurance Standards for AEFI surveillance Roll out of the QMS in the States, budget included in the PIP, trainings and internal assessment started at state, district and PHC/session site levels
- 4. 01 02 03 04 Areas of Concern Standard Measurable Elements Score card (Session Site, District, State and National) Measurable Elements Standard 8 40 54 Framework 4
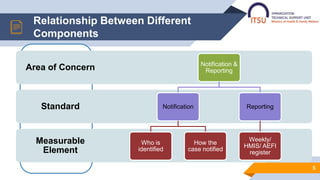
- 5. Relationship Between Different Components 5 Measurable Element Standard Area of Concern Notification & Reporting Notification Who is identified How the case notified Reporting Weekly/ HMIS/ AEFI register
- 6. NQAS for AEFI Surveillance checklists ? National level checklist ? State level checklist ? District level checklist ? Immunization site level checklist 6
- 7. Areas of Concern 7 S. No Area of Concern Immunization site District State National A Notification & Reporting Y Y Y Y B Investigation Y Y Y Y C Causality Assessment NA NA Y Y D Operational Management Y Y Y Y E Communication Y Y Y Y F Convergence Y Y Y Y G Monitoring & Feedback NA Y Y Y H Quality Management System Y Y Y Y
- 8. Area of concern in NQAS and QMS for AEFI Surveillance 8 S. No Areas of concern in NQAS for public health facilities Areas of concern in AEFI Surveillance A Service Provision Notification and Reporting B Patient rights Investigation C Inputs Causality Assessment D Support services Operational Management E Clinical services Communication F Infection control Convergence G Quality Management Monitoring and Feedback H Outcome Quality Management System To assess the quality system under NQAS and AEFI surveillance are broadly categorized into measurable themes known as “Area of Concerns”. The areas of concern in NQAS for public health facilities and QMS in AEFI surveillance are different, as can be seen from the table below:
- 9. Assessment protocol 9 OBSERVATION (OB) STAFF INTERVIEW (SI) RECORD REVIEW (RR) PARENT INTERVIEW (PI)
- 10. Scoring Rules 10 ? 2 marks for full compliance ? 1 mark for partial compliance ? 0 Marks for Non Compliances
- 12. Score card 12 National Quality Assurance Standards for AEFI Surveillance Programme Checklist for Immunization Sites Assessment Summary Name of the Immunization sites: PHC PALANA Date of Assessment:14/04/2023 Names of Assessors: Dr.Ravit Patel Names of Assesses: K N Pateliya Type of Assessment: Internal Action plan Submission Date : 15/04/2023 Immunization Sites Score Card Area of Concern wise Score Total Score A Notification & Reporting 91% 72% B Investigation 100% D Operational Management 77% E Communication 40% F Convergence 0% H Quality Management System 62%
- 13. Eligibility criteria for PHC/ Session site level assessment 13 Internal Assessment Internal assessment should be done quarterly by Medical Officer of the PHC. PHC becomes eligible for peer assessment if 50% of the sub centre score at least 70% in the internal assessment. Peer Assessment Peer assessment of a PHC/Sub- Center will be conducted by the Medical Officer of another PHC of the same district. Peer assessment will be done for the PHC and for those sub- centres under the PHC which have scored at least 70% in internal assessment. External Assessment NA
- 14. Eligibility criteria for District level assessment 14 Internal Assessment DIO/District Quality Assessment Team will conduct internal assessment for district level. District will be eligible for peer assessment when the district level achieves at least 70% score in internal assessment and 50% of district PHCs score at least 70% in internal assessment. Peer Assessment Peer assessment of the district will be done by District Immunization Officer/District Quality Assurance Team of another district. Peer assessor will validate internal assessment scores of district level and also validate two PHCs and one sub centre in each of the two PHCs scoring at least 70%. External Assessment External Assessment will be done for the district, if the district scores at least 70% during peer assessment and 50% of PHCs score at least 70% during peer assessment. External assessment will be done by certified external assessors from NHSRC, MoHFW.
- 15. Eligibility criteria for State level assessment 15 Internal Assessment State Immunization Officer or State Quality Assurance cell will conduct the internal assessment of state. If state achieves at least 70% score in internal assessment and 50% of the districts in the state will get 70% score in peer assessment then the State will be eligible for peer assessment. Peer Assessment Peer assessment of the state will be done by State Immunization Officer/State Quality Assurance Team of another state/AEFI Secretariat. External Assessment State will be eligible for external assessment if the state level scores at least 70% during peer assessment and 50% districts of the state scores at least 70% during peer assessment. External assessment will be done by external assessors certified by NHSRC MOHFW.
- 16. Responsibility of PHC/District/State level officers 16 Responsibility of Medical Officer/ District Immunization Officer/District Quality Team 1. Medical officer will review checklists and gap action plans following quarterly internal assessment of the sub-centre and ensure implementation of action plans for gap closure. 2. Medical officer, PHC will share internal assessment scores and gap improvement plans of the PHC and the sub-centre immunization sites to the District Immunization Officer for each quarter. 3. Peer assessment scores of PHC and sub-centre immunization sites will be shared by the assessor with the district. Responsibility of District Immunization Officer/District Quality Team 1. District Immunization Officer/District Quality Team will conduct an internal assessment for district level and collate and verify scores of internal and peer assessment of PHCs. 2. Plans for peer assessment of PHCs/sub-centre will be prepared and implemented by District Immunization Officer. 3. Peer assessor from another district will share the assessment report to the state with a copy to the District Immunization Officer of the assessed district. Responsibility of State Immunization Officer/State Quality Team 1. State Immunization Officer/State Quality Assurance cell will collate and verify district-level internal and peer assessment scores of all districts. 2. State Immunization Officer/State Quality Assurance Cell will plan and coordinate peer assessment of a district by another district.
- 17. Process of QMS for AEFI Surveillance ? At the PHC level or session site levels, the focus of the checklists to assess awareness of reporting criteria of AEFIs, documentation of AEFI Cases, awareness of safety aspects in session sites, and communication-related to vaccine safety to beneficiaries, feedback from beneficiaries, and support to be provided during investigations. ? At the district levels, the focus is mainly on processes and documentation related to AEFI surveillance at the DIO’s office, data entry and uploading AEFI case records and reports in SAFE-VAC, conducting meetings of the District AEFI Committee, coordination and convergence with other stakeholders such as district hospitals, private hospitals, nursing homes, and practitioners, drug inspectors and faculty from the medical college, if any, and conducting AEFI investigations. ? At the state level, the documentation of cases, reviewing of AEFI surveillance, conducting causality assessments by state, AEFI committee meetings, coordination with the State Drug Controller, medical colleges, private sector, etc.
- 18. Approved budgetary norms and assessment criteria ? A budget head created for QMS for AEFI surveillance activities under UIP. ? Budgetary norms for each activity in state and district PIPs ? Implementation at state, district, session sites (PHC, sub centres) simultaneously ? Roles of immunization programme managers and quality assurance officers defined at each levels ? Roles of specific health cadres (SEPIOs, DIOs, MOs, and ANMs) specified
- 19. Steps for Assessment and Certification Internal Assessment ? Assessment is done using a checklist by a team of the same facility Peer Assessment ? Assessment is done by a team from another facility of the same level after the facility/level achieves the necessary scores in the internal assessment External Assessment ? Done by trained experts empanelled by QI Division of NHSRC for state
- 20. Implementing guidebook of QMS for AEFI Surveillance in States and Districts ? This document has been prepared to help the state and district immunization Programme officers to initiate QMS processes rapidly in the state and districts to attain certification as per NQAS for AEFI surveillance processes. 20
- 21. Implementation Status of Quality Management System- QMS 21 *Provisional data as of May 2023 RAJ AS THAN LADAKH OD ISHA GU JAR AT MAHARAS HTRA MADHY A P RADE SH BIHAR KAR NATAKA UTTAR P RADES H AS SAM TAMI L NAD U TE LAN GANA AND HRA PRADE SH PUNJ AB JHARKHAND WE ST BE NG AL ARU NAC HAL P R. HAR YANA KERALA JAM MU & KASH MIR HIMACHAL P RAD ES H MANIP UR MIZO RAM SIKK IM A&N ISLANDS D&N HAV ELI CHHATTIS G AR H UTTARAKHAND ME GH ALAYA NAG ALAN D TRI PURA GO A DELHI CHANDIG ARH DAM AN & DIU PO NDIC HERR Y LAKS HADW EE P State/UT selected for 1st Phase (FY-2021-22) State/Ut selected for 2nd Phase (FY-2022-23) State/Ut selected for 3rd Phase (FY-2023-24) Phase-1 Phase-2 Phase-3 ASSAM A&N ISLANDS ANDHRA PRADESH BIHAR CHHATTISGARH ARUNACHAL PRADESH CHANDIGARH DAMAN & DIU JAMMU & KASHMIR GOA D&N HAVELI JHARKHAND GUJARAT DELHI LADAKH HARYANA LAKSHADWEEP MADHYA PRADESH HIMACHAL PRADESH MANIPUR MAHARASHTRA KARNATAKA MIZORAM PUNJAB KERALA NAGALAND RAJASTHAN MEGHALAYA PUDUCHERRY UTTAR PRADESH ODISHA SIKKIM UTTARAKHAND TELANGANA TAMIL NADU WEST BENGAL TRIPURA
- 22. Thank you
Editor's Notes
- #12: A-The title of the checklist denotes the name of the level for which the checklist is intended. B-Extreme left column of checklist contains the reference number of the standard and Measurable Elements. C-The row in grey colour contains the name of the area of concern under which the standards are listed. D-The row in yellow colour horizontal bar has statement describing the standard which is being measured. There are a total of forty standards. E-The second column contains text of the measurable elements for the respective standard. Only applicable measurable elements of a standard are shown in a checklist. F-A blank column to the right of the measurable element is the space to record findings of assessment in terms of compliance, partial and non-compliance G-The column to the right of the blank compliance column is the assessment method column. It explains the ‘HOW TO’ to gather the information.