Packaging materials : Selection & Evaluation
- 1. SELECTION AND EVALUATION OF PHARMACEUTICAL PACKAGING MATERIALS PRESENTED BY : YASH R.MENGHANI M.PHARM 1ST YEAR (QA) SMT.KISHORITAI BHOYAR COLLEGE OF PHARMACY,KAMPTEE 1
- 2. PACKAGING : ’üĄ Packaging Can Be Defined As An Economical Means Of Providing Presentation, Protection, Identification Information, Convenience And Compliance For A Product During Storage, Carriage, Display And Until The Product Is Consumed. ’üĄ Packaging Must Provide Protection Against Climatic Conditions Biological, Physical And Chemical Hazards And Must Be Economical. The Package Must Ensure Adequate Stability Of The Product Throughout The Shelf Life. 2
- 3. SELECTION OF PACKAGING MATERIALS ACCORDING TO CONTAINERS : ’üĄ Selection Of Packaging Materials According To Containers Glass & Plastic ’üĄ Selection Of Packaging Materials According To Containers Metal,Closures ’üĄ Selection Of Packaging Materials According To Product Characteristics 3
- 4. CHARACTERISTICS OF PACKAGING MATERIALS:- ’üĄ They must protect the preparation from environmental conditions. ’üĄ They must not be reactive with the product. ’üĄ They must not impart to the product tastes or odours. ’üĄ They must be nontoxic. 4
- 5. CHARACTERISTICS OF PACKAGING MATERIALS:- ’üĄ They must be FDA approved. ’üĄ They must meet applicable tamper-resistance requirements. ’üĄ They must be adaptable to commonly employed high speed packaging equipment. 5
- 6. SELECTION OF THE PACKAGING MATERIALS ’üĄ On the facilities available, for example, pressurized dispenser requires special filling equipment. ’üĄ On the ultimate use of product. The product may be used by skilled person in hospital or may need to be suitable for use in the home by a patient. ’üĄ On the physical form of the product. For example, solid, semi-solid, liquids or gaseous dosage form. ’üĄ On the route of administration. For example, oral, parenteral, external, etc. 9 6
- 7. SELECTION OF THE PACKAGING MATERIALS ’üĄ On the stability of the material. For example , moisture, oxygen, carbon di oxide, light, trace metals, temperature or pressure or fluctuation of these may have a deleterious effect on the product. ’üĄ On the contents. The product may react with the package such as the release of alkali from the glass or the corrosion of the metals and intern the product is affected. ’üĄ On the cost of the product. Expensive products usually justify expensive packaging 7
- 8. FACTORS AFFECTING SELECTION OF PACKAGING MATERIALS Mechanical Factors:- ’üĄ Shock ’üĄ Compression ’üĄ Puncture ’üĄ Vibration 8
- 9. FACTORS AFFECTING SELECTION OF PACKAGING MATERIALS Environmental Factors:- ’üĄ Temperature ’üĄ Pressure ’üĄ Moisture ’üĄ Gases ’üĄ Light ’üĄ Infestation ’üĄ Contamination 9
- 10. CONTAINERS ’üĄ Container is one in which the product is placed. ’üĄ A pharmaceutical container is defined as a device that holds the drugs and is or may be in direct contact with the preparation. 10
- 11. IDEAL REQUIREMENTS OF CONTAINERS ’üĄ Able to withstand changes in pressure and temperature. ’üĄ Must be non-toxic. ’üĄ Can be labelled easily. ’üĄ Pharmaceutically elegant appearance. 11
- 12. TYPES OF CONTAINERS ’üĄ Well-closed containers ’üĄ Single dose containers ’üĄ Multi dose containers ’üĄ Light-resistant containers ’üĄ Air-tight containers ’üĄ Aerosol containers 12
- 13. MATERIALS USED FOR MAKING OF CONTAINERS ’üĄ 1. Glass ’üĄ 2. Plastic ’üĄ 3. Metal ’üĄ 4. Paper and board 13
- 14. ADVANTAGES OF GLASS CONTAINERS : ’üĄ They Are Transparent. ’üĄ They Are Available In Various Shapes And Sizes. ’üĄ They Can Withstand The Variation In Temperature And Pressure During Sterilization. ’üĄ They Are Economical And Easily Available. ’üĄ They Can Protect The Photosensitive Medicaments From Light During Their Storage. ’üĄ They Have Good Protection Power. ’üĄ They Can Be Easily Labelled. 14
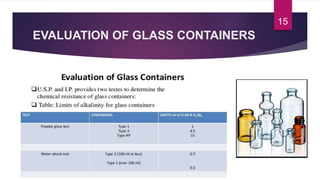
- 15. EVALUATION OF GLASS CONTAINERS 15
- 16. EVALUATION OF GLASS CONTAINERS ARSENIC TEST : Washed the inner and outer surface of container with fresh distilled water for 5min. Prep test as described in the test for hydrolytic resistance for an adequate no. of samples to produce 50ml. pipette out 10ml solution from combined contents of all ampoules to the flask. Add 10ml of HNO3 to dryness on the water bath, dry the residue in an oven at 130Ōü░C for 30min cool. Swirl to dissolve and heat under water bath and reflux for 25min. Cool to room temp and determine the absorbance at 840nm. The absorbance of the test solution should not exceed the absorbance obtained by repeating the determination using 0.1ml of arsenic standard solution (10ppm) in place of test solution 16
- 17. ADVANTAGES OF PLASTIC CONTAINERS ’üĄ They are light in weight and can be handled easily. ’üĄ They are poor conductor of heat. ’üĄ They can be transported easily. ’üĄ They are unbreakable. ’üĄ They are available in various shapes and sizes. ’üĄ They have good protection power. 17
- 18. EVALUATION OF PLASTICS:- ’üĄ Leakage test ’üĄ Collapsibility test ’üĄ Water vapor permeability test 18
- 19. EVALUATION OF PLASTICS:- 1.Leakage Test : Fill 10 containers with water, fit with intended closures and keep them inverted at room temperature for 24hr. The test is said to be passed if there is no signs of leakage from any container. 2. Collapsibility Test : This test is applicable to the containers which are to be squeezed for removing the contents. A container by collapsing inward during use, yield at least 90% of its normal contents at the required rate of flow at ambient temperature. 19
- 20. EVALUATION OF PLASTICS:- 3.Water Vapour Permeability Test : Fill 5 containers with normal volume of water and heat seal the bottles with an aluminium foil. Weigh accurately each container and allowed to stand for 14days at temperature between 20 and 25Ōü░C. Reweigh the containers. 20
- 21. ADVANTAGES OF METAL CONTAINERS ’üĄ They are sturdy. ’üĄ They are impermeable to light, moisture and gases. ’üĄ They can be made into rigid unbreakable containers by impact extrusion. ’üĄ They are light in weight as compared to glass containers 21
- 22. CLOSURES ’üĄ Closures are the devices by means of which containers can be opened and closed. ’üĄ It prevents loss of material by spilling or volatilization. ’üĄ It avoids contamination of the product from dirt, micro- organism or insects. ’üĄ It prevents deterioration of the product from the effect of the environment such as moisture, oxygen or carbon dioxide. 22
- 23. EVALUATION OF CLOSURES:- ’üĄ 1. Sterilization test ’üĄ 2. Fragmentation test ’üĄ 3. Self Sealibility test 23
- 24. EVALUATION OF CLOSURES:- 1.FRAGMENTATION TEST (IP 1996) : Place A Volume Of Water Corresponding To Nominal Volume-4ml In Each Of 12 Clean Vials, Close Vial With Closure And Secure Caps For 16hrs. Pierce The Closure With Number 21 Hypodermic Needle and Inject 1ml Water And Remove 1ml Air Repeat The Above Operation 4 Times For Each Closure Count The Number Of Fragments Visible To Naked Eye Total Number Of Fragments Should Not Be More Than 10. 24
- 25. EVALUATION OF CLOSURES:- 2.SELF SEALABILITY TEST FOR RUBBER CLOSURES : Fill 10 Vials With Water To Small Volume And Close The Vials With Closures, Pierce The Cap And Closures 10 Times At Different Places With No 21 Syringe Needle. Immerse The Vials In 0.1 %W/V Solution Of Methylene Blue Under Reduced Pressure, Restore The Nominal Pressure And Keep The Container For 30 Min And Wash The Vials. None Of The Vial Should Contain Traces Of Colored Solution. 25
- 26. 26