Pcr presentation Dr,Kamlesh shah
- 2. Introduction • PCR, polymerase chain reaction, is an in-vitro technique for amplification of a region of DNA whose sequence is known or which lies between two regions of known sequence • Before PCR, DNA of interest could only be amplified by over-expression in cells and this with limited yield
- 3. History 1. 1966, Thomas Brock discovers Thermus Aquaticus, a thermostable bacteria in the hot springs of Yellowstone National Park 2. 1983, Kary Mullis postulated the concept of PCR ( Nobel Prize in 1993) 3. 1985, Saiki publishes the first application of PCR ( beta-Globin) 4. 1985, Cetus Corp. Scientists isolate Thermostable Taq Polymerase (from T.aquaticus), which revolutionized PCR
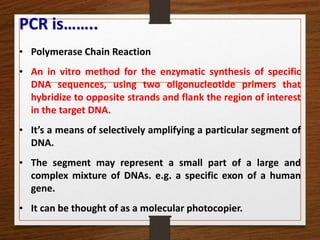
- 4. PCR is…….. • Polymerase Chain Reaction • An in vitro method for the enzymatic synthesis of specific DNA sequences, using two oligonucleotide primers that hybridize to opposite strands and flank the region of interest in the target DNA. • It’s a means of selectively amplifying a particular segment of DNA. • The segment may represent a small part of a large and complex mixture of DNAs. e.g. a specific exon of a human gene. • It can be thought of as a molecular photocopier.
- 5. Principle… • An in vitro method for enzymatic synthesis of DNA • Reaction uses two oligonucleotide primers that hybridize to opposite strands and flank the region of interest. • A heat stable DNA polymerase catalyses the elongation of primers. • Primers extension products serve as template in next cycle. • Numbers of target copies double in each cycle.
- 6. 6 What’s Requirement for PCR? Genomic DNA 5’ 3’ 3’ 5’ primers A B Free nucleotides Taq DNA polymerase Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Mg2+ Buffer containing magnesium
- 7. The Basic Protocol--Denaturation Genomic DNA 95oC 5’ 3’ 3’ 5’ 5’ 3’ 3’ 5’
- 8. The Basic Protocol--Annealing ~55oC 5’ 3’ 3’ 5’ 5’ 3’ 3’ 5’ 5’ 5’ Genomic DNA A B primers A B
- 9. The Basic Protocol-Extension 72oC 5’ 3’ 3’ 5’ 5’ 3’ 3’ 5’ 5’ 5’ Genomic DNA Taq polymerase
- 10. Former DNA amplification in vitro • Water baths- Three different temperature 92-94 º C 55 º C 74 º C • Tubes were moved physically-manually • Large volume, More chemical - Costly • Klenov fragment used to amplify DNA (DNA polymerase from E. coli)
- 11. PCR Reaction Condition Steps Temperature Time Denaturing 94 o C 20-30 sec Annealing 55o C 20-60 sec Extension 72 o C 30-60 sec 72oC Extending 94oC Denaturizing 55oC Annealing
- 12. 1- DNA template • DNA containing region to be sequenced • Size of target DNA to be amplified : up to 3 Kb
- 13. 2- Primers • 2 sets of primers • Generally 20-30 nucleotides long • Synthetically produced • complimentary to the 3’ ends of target DNA • not complimentary to each other
- 14. Primers (ctnd) • Not containing inverted repeat sequences to avoid formation of internal structures • 40-60% GC content preferred for better annealing • Tm of primers can be calculated to determine annealing T0 • Tm= .41(%G+C) + 16.6log(J+) + 81.5 where J+ is the concentration of monovalent ions
- 15. 3-Enzyme • Usually Taq Polymerase or anyone of the natural or Recombinant thermostable polymerases • Stable at T0 up to 950 C • High processivity • Taq Pol has 5’-3’ exo only, no proofreading
- 22. RT-PCR • Reverse Transcriptase PCR • Uses RNA as the initial template • RNA-directed DNA polymerase (rTh) • Yields ds cDNA
- 26. Detection of amplification products • Gel electrophoresis • Sequencing of amplified fragment • Southern blot • etc...
- 27. 27 • Aims to reduce nonspecific background by gradually lowering the annealing temperature as PCR cycling progresses. • The annealing temperature at the initial cycles is usually a few degrees above the Tm of the primers used, while at the later cycles, it is a few degrees below the primer Tm • The higher temperatures give greater specificity for primer binding, and the lower temperatures permit more efficient amplification from the specific products formed during the initial cycles. • This ensures that only specific annealing of the primers to their correct target sequence takes place before any non specific annealing events occur Touch Down PCR
- 28. • Two pairs of PCR primers for a single locus in two successive reactions. • First reaction- one pair to generate DNA products (consist non- specifically amplified DNA fragment) • Second reaction- with a set of primers whose binding sites are completely within the DNA target fragment • The second pair of primers (nested primers) bind within the first PCR product and produce a second PCR product shorter than the first one • If the wrong locus were amplified by mistake, the probability is very low that it would also be amplified a second time by a second pair of primers. • Increases the specificity of DNA amplification, by reducing background due to non-specific amplification of DNA. Nested PCR
- 29. 29 Enables simultaneous amplification of many targets of interest in one reaction Multiple, unique primer sets within a single PCR reaction to produce amplicons of varying sizes specific to different DNA sequences. By targeting multiple genes at once, additional information may be gained from a single test run that otherwise would require several times the reagents and more time to perform. Annealing temperatures for each of the primer sets must be optimized to work correctly within a single reaction, and amplicon sizes, i.e., their base pair length, should be different enough to form distinct bands when visualized by gel electrophoresis Multiplex PCR
- 30. Applications of PCR Basic Research Applied Research • Genetic matching • Detection of pathogens • Pre-natal diagnosis • DNA fingerprinting • Gene therapy • Mutation screening • Drug discovery • Classification of organisms • Genotyping • Molecular Archaeology • Molecular Epidemiology • Molecular Ecology • Bioinformatics • Genomic cloning • Site-directed mutagenesis • Gene expression studies
- 31. Applications of PCR Molecular Identification Sequencing Genetic Engineering • Molecular Archaeology • Molecular Epidemiology • Molecular Ecology • DNA fingerprinting • Classification of organisms • Genotyping • Pre-natal diagnosis • Mutation screening • Drug discovery • Genetic matching • Detection of pathogens • Bioinformatics • Genomic cloning • Human Genome Project • Site-directed mutagenesis • Gene expression studies
- 34. Detection of Unknown Mutations Molecular Identification:
- 35. Classification of Organisms 1) Relating to each other 2) Similarities 3) Differences * Fossils * Trace amounts * Small organisms ! DNA ! Molecular Identification: Insufficient data
- 36. Detection Of Pathogens Molecular Identification:
- 37. Prenatal Diagnosis 644 bp 440 bp 204 bp Molecular analysis of a family with an autosomal recessive disease. Molecular Identification: • Chorionic Villus • Amniotic Fluid
- 38. SEQUENCING Nucleotides (dNTP) are modified (dideoxynucleotides = ddNTP) NO polymerisation after a dideoxynucleotide! Fragments of DNA differing only by one nucleotide are generated Nucleotides are either or
- 39. Applications • Genome mapping and gene function determination • Biodiversity studies ( e.g. evolution studies) • Diagnostics ( prenatal testing of genetic diseases, early detection of cancer, viral infections...) • Detection of drug resistance genes • Forensic (DNA fingerprinting)
- 40. Advantages • Automated, fast, reliable (reproducible) results • Contained :(less chances of contamination) • High output • Sensitive • Broad uses • Defined, easy to follow protocols
- 41. Conclusion The speed and ease of use, sensitivity, specificity and robustness of PCR has revolutionised molecular biology and made PCR the most widely used and powerful technique with great spectrum of research and diagnostic applications.
- 42. References • Fundamentals of Biochem ( Voet, Voet, Pratt) • Molecular Cell Biology ( Lodish, Darnell..)