PELLETS - BASIC AND COMPOSITION UNIT DOSAGE FORM
- 1. ¡° PELLETS - BASIC AND COMPOSITION UNIT DOSAGE FORM ¡± Submitted By: Raj Kumar Mandal B. Pharm, Vth Sem, ISFCP, Moga INDUSTRIAL PHARMACY-I: THEORY Submitted To: Mr. Rahul Pal *Assistant Professor, Department of Pharmaceutics, ISF College of Pharmacy, Moga, Punjab, India
- 2. INTRODUCTION: PELLTS ? Pellets are small, free-flowing, spherical or semi-spherical solid particles usually within the size range of 0.5¨C2 mm. They are designed for oral drug delivery and are often used as a multi- particulate dosage form in the pharmaceutical industry. Applications ?Modified Release Systems: Often used in controlled, sustained, or delayed-release drug formulations. ?Combination Therapy: Different drugs can be incorporated into separate pellets within a single capsule, allowing combination therapy in one dosage form. ?Targeted Delivery: Pellets can be coated with polymers for targeted delivery to specific areas in the GI tract. Common Manufacturing Techniques ?Extrusion-Spheronization: Commonly used for high drug-loading formulations. ?Layering Techniques: Involves layering active ingredients onto inert cores. ?Spray Congealing and Spray Drying: Used for temperature-sensitive drugs or to create unique release profiles.
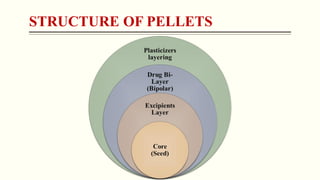
- 4. CONT...... 1. Core (Seed): ? It act as a drug receiver, which may be inert in natured in some cases and improve the mechanical strength of Pellets. 2. Excipients (Fillers, Binder, Glidant, Lubricant): ? It's used to improve quality, drug release pattern and enhancement of mechanical properties. 3. Drug Layer: ? As per requirement API will select for specific disease target. 4. Plasticizer: ? It enhance the flexibility of pellets coating (e.g. Polethylene Glycol) and prevention of oxidation for which antioxidant are used (e.g. Tocopheron).
- 5. STEPS OF PELLETS FORMULATION 1. Weighing and Mixing ? The specified selected powder/API according weight and mixed properly by a blinder. 2. Wetting Mass/ Dough Formation ? A binder solution is added to form dough for pellets formation. 3. Extrusion ? To form cylindrical small extruded for further spheronization process. 4. Spheronization ? In this, the prepared extrudes round in uniform and formed spherical Pellets by spheronizer.
- 6. CONT..... 5. Drying ? Pellets are dried for achieving the stable moisture content and final pellets formulation for further packing in a hard gelatin capsules. 6. Coating layer (Optional) ? As per drug release pattern, coating may be applied on pellets.
- 7. PELLETIZATION PROCESS ? In this process of pharmaceutical formulations to create small free flowing and spherical unit called pellets. ? Pelletization objective is to improve drug release properly, enhancement of bioavailability and control & immediately drug release of drug. Mixing ¡ú Extrudes ¡ú Spheronization ¡ú Drying ¡ú Coating ¡ú Packaging
- 8. EQUIPMENTS INVOLVED IN PELLETIZATION PROCESS ? 1. Blender/Granulator ? 2. Extruder ? 3. Spheronizer ? 4. Fluidized Bed Dryer ? 5. Sieves Shaker
- 9. METHOD OF PREPARATION OF PELLETS
- 10. METHOD OF PREPARATION OF PELLETS ? 1. Extrusion Spheronization ? It is widely used for lab purpose. ? This method used for high drug dose loading & obtained maximum uniform size pellets. ? Procedure - Mixing ¡ú Extrusion ¡ú Spheronization ¡ú Drying ¡ú Coating¡ú...........
- 11. CONT..... 2. Layering Method ? (i) Power Layering/Drawing ? This method effective for low dosage formulation and mostly used for test masking purpose. Procedure ?Step 1: Core Preparation: Neutral cores (sugar spheres or microcrystalline cellulose) are placed in a fluidized bed or coating pan. ?Step 2: Adhesive Application: A binding solution is sprayed onto the cores to make the surface tacky, allowing powder to adhere effectively. ?Step 3: Powder Application: Fine drug powder or excipient powder is introduced and adheres to the wet, tacky surface of the cores. ?Step 4: Drying and Layering Cycles: Alternating cycles of binding solution application and powder layering continue until the desired pellet size and drug loading are achieved. ?Step 5: Final Drying: Pellets are dried thoroughly to stabilize the layered material and prevent powder loss during handling.
- 12. CONT.... 3. Rotary Processing ? This method used are centrifugal force in a rotating chamber to form pellets mixture of powders with binding agents. Procedure ?Step 1: Loading the Drum: Neutral cores (like sugar or microcrystalline cellulose beads) are placed in the rotating drum. ?Step 2: Spraying Binding Solution: A binding solution is sprayed onto the rotating cores to make them tacky, allowing powdered material to adhere and build layers. ?Step 3: Powder Application: Drug powder or excipient is added, adhering to the tacky surface created by the binding solution. ?Step 4: Layering Cycles: Alternating cycles of spraying and powder addition continue until the desired pellet size and drug loading are achieved. ?Step 5: Drying: Pellets are dried in the rotating drum or transferred to a drying unit to stabilize the layers.
- 13. CONCLUSION ? The study of pellets as a pharmaceutical dosage form highlights their significant advantages and versatility in drug delivery systems. Key findings from this research indicate that pellets exhibit improved bioavailability due to their increased surface area, which allows for better dissolution and absorption of the drug in the gastrointestinal tract. ? This results in more stable plasma drug concentrations and reduced dosing frequency. By distributing the drug more evenly throughout the gastrointestinal tract, pellets also minimize localized irritation, enhancing patient comfort and adherence to treatment. ? Future research should focus on innovative materials and techniques to further enhance the functionality and performance of pellet-based systems. In summary, pellets represent a promising approach in modern pharmaceutical formulations, offering enhanced drug delivery capabilities, patient compliance, and potential for improved therapeutic outcomes.
- 14. REFERENCES 1. Xu M, Sun M, Qiao H, Ping Q, Elamin ES. Preparation and evaluation of colon adhesive pellets of 5-aminosalicylic acid. International Journal of Pharmaceutics. 2014 Jul 1;468(1- 2):165-71. 2. Gajdziok J, Bernatoniene J, Musel¨ªk J, Masteikov¨¢ R, Dvo?¨¢?kov¨¢ K, Petkeviciute Z, Lazauskas R, Kalveniene Z, Bernatoniene R. The evaluation of formulations for the preparation of new formula pellets. Pharmaceutical development and technology. 2011 Oct 1;16(5):520-8. 3. Pal, Rahul & Mandal, Raj. (2024). Preparation and evaluation parameters of PCM loaded pellets using Extrusion Spheronization [Experiment Findings]. 10.13140/RG.2.2.25721.07525. 4. Akhgari A, Abbaspour MR, Pirmoradi S. Preparation and evaluation of pellets using acacia and tragacanth by extrusion-spheronization. DARU Journal of Pharmaceutical Sciences. 2011;19(6):417.













![REFERENCES
1. Xu M, Sun M, Qiao H, Ping Q, Elamin ES. Preparation and evaluation of colon adhesive
pellets of 5-aminosalicylic acid. International Journal of Pharmaceutics. 2014 Jul 1;468(1-
2):165-71.
2. Gajdziok J, Bernatoniene J, Musel¨ªk J, Masteikov¨¢ R, Dvo?¨¢?kov¨¢ K, Petkeviciute Z,
Lazauskas R, Kalveniene Z, Bernatoniene R. The evaluation of formulations for the
preparation of new formula pellets. Pharmaceutical development and technology. 2011 Oct
1;16(5):520-8.
3. Pal, Rahul & Mandal, Raj. (2024). Preparation and evaluation parameters of PCM loaded
pellets using Extrusion Spheronization [Experiment Findings].
10.13140/RG.2.2.25721.07525.
4. Akhgari A, Abbaspour MR, Pirmoradi S. Preparation and evaluation of pellets using acacia
and tragacanth by extrusion-spheronization. DARU Journal of Pharmaceutical Sciences.
2011;19(6):417.](https://image.slidesharecdn.com/pelletstheory-241029092538-69a89c8d/85/PELLETS-BASIC-AND-COMPOSITION-UNIT-DOSAGE-FORM-14-320.jpg)
