Periodic table review
- 1. + Periodic Table Review Chemistry 101 Miss McClelland
- 2. + History
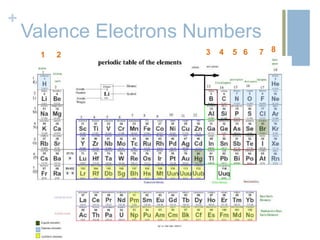
- 7. + Valence Electrons Numbers 1 6 7 8542 3
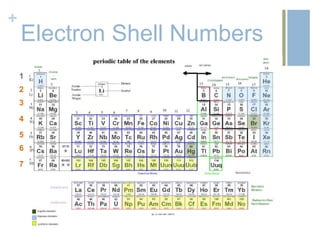
- 9. +
Editor's Notes
- Dmitri Mendeleev and Lothar Meyer both developed and published an arrangement of Elements in 1869. The Elements were arranged by chemical and physical properties, and atomic weight. Henry Moseley added Atomic numbers to the Elements in 1913.