Pharmaceutical chemistry
- 1. ULTRAVIOLET/VISIBLE ABSORPTION SPECTROSCOPY Widely used in chemistry. Perhaps the most widely used in Biological Chemistry. Easy to do. Very easy to do wrong. Dr.Samer HOUSHEH
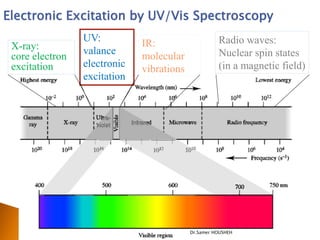
- 2. Electronic Excitation by UV/Vis Spectroscopy UV: Radio waves: X-ray: IR: valance Nuclear spin states core electron molecular excitation electronic (in a magnetic field) vibrations excitation Dr.Samer HOUSHEH
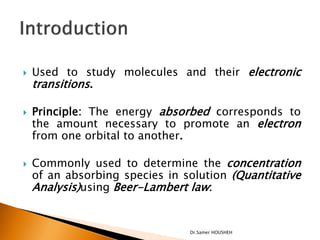
- 3. ’üĮ Used to study molecules and their electronic transitions. ’üĮ Principle: The energy absorbed corresponds to the amount necessary to promote an electron from one orbital to another. ’üĮ Commonly used to determine the concentration of an absorbing species in solution (Quantitative Analysis)using Beer-Lambert law: Dr.Samer HOUSHEH
- 4. The wavelength and amount of light that a compound absorbs depends on its molecular structure and the concentration of the compound used. The concentration dependence follows BeerŌĆÖs Law. A=ebc = log I/I0 Where A is absorbance e is the molar absorptivity with units of L mol-1 cm-1 b is the path length of the sample (typically in cm). c is the concentration of the compound in solution, expressed in mol L-1 Dr.Samer HOUSHEH
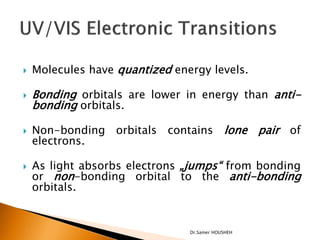
- 5. ’üĮ Molecules have quantized energy levels. ’üĮ Bonding orbitals are lower in energy than anti- bonding orbitals. ’üĮ Non-bonding orbitals contains lone pair of electrons. ’üĮ As light absorbs electrons ŌĆ×jumpsŌĆ£ from bonding or non-bonding orbital to the anti-bonding orbitals. Dr.Samer HOUSHEH
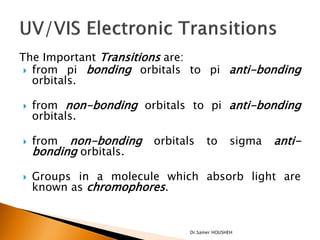
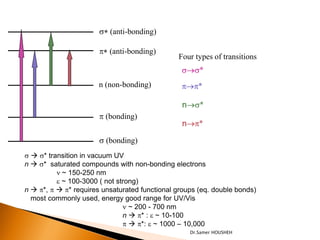
- 6. The Important Transitions are: ’üĮ from pi bonding orbitals to pi anti-bonding orbitals. ’üĮ from non-bonding orbitals to pi anti-bonding orbitals. ’üĮ from non-bonding orbitals to sigma anti- bonding orbitals. ’üĮ Groups in a molecule which absorb light are known as chromophores. Dr.Samer HOUSHEH
- 7. s* (anti-bonding) p* (anti-bonding) Four types of transitions s’é«s* n (non-bonding) p’é«p* n’é«s* p (bonding) n’é«p* s (bonding) s ’āĀ s* transition in vacuum UV n ’āĀ s* saturated compounds with non-bonding electrons n ~ 150-250 nm e ~ 100-3000 ( not strong) n ’āĀ p*, p ’āĀ p* requires unsaturated functional groups (eq. double bonds) most commonly used, energy good range for UV/Vis n ~ 200 - 700 nm n ’āĀ p* : e ~ 10-100 p ’āĀ p*: e ~ 1000 ŌĆō 10,000 Dr.Samer HOUSHEH
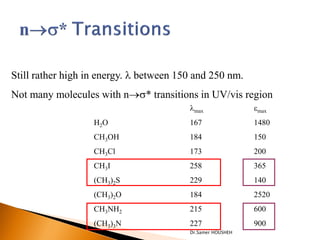
- 8. Still rather high in energy. ’ü¼ between 150 and 250 nm. Not many molecules with n’é«s* transitions in UV/vis region ’ü¼max emax H2O 167 1480 CH3OH 184 150 CH3Cl 173 200 CH3I 258 365 (CH3)2S 229 140 (CH3)2O 184 2520 CH3NH2 215 600 (CH3)3N 227 900 Dr.Samer HOUSHEH
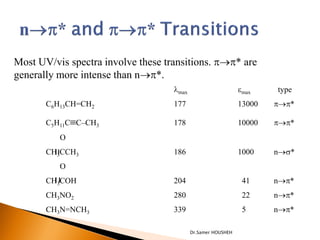
- 9. Most UV/vis spectra involve these transitions. p’é«p* are generally more intense than n’é«p*. ’ü¼max emax type C6H13CH=CH2 177 13000 p’é«p* C5H11C’é║CŌĆōCH3 178 10000 p’é«p* O CH3CCH3 186 1000 n’é«s* O CH3COH 204 41 n’é«p* CH3NO2 280 22 n’é«p* CH3N=NCH3 339 5 n’é«p* Dr.Samer HOUSHEH
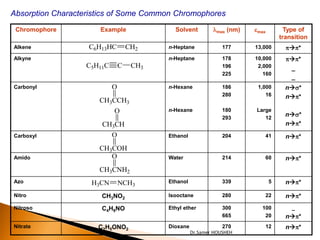
- 10. Absorption Characteristics of Some Common Chromophores Chromophore Example Solvent ’ü¼max (nm) emax Type of transition Alkene C6H13HC CH2 n-Heptane 177 13,000 p’āĀp* Alkyne n-Heptane 178 10,000 p’āĀp* C5H11C C CH3 196 2,000 _ 225 160 _ Carbonyl O n-Hexane 186 1,000 n’āĀs* 280 16 n’āĀp* CH3CCH3 O n-Hexane 180 Large 293 12 n’āĀs* CH3CH n’āĀp* Carboxyl O Ethanol 204 41 n’āĀp* CH3COH Amido O Water 214 60 n’āĀp* CH3CNH2 Azo H3CN NCH3 Ethanol 339 5 n’āĀp* Nitro CH3NO2 Isooctane 280 22 n’āĀp* Nitroso C4H9NO Ethyl ether 300 100 _ 665 20 n’āĀp* Nitrate C2H5ONO2 Dioxane 270 12 n’āĀp* Dr.Samer HOUSHEH
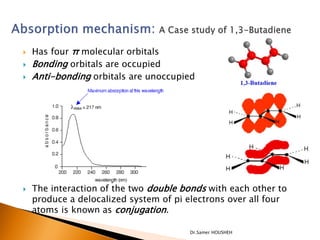
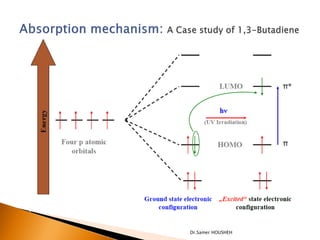
- 11. ’üĮ Has four ŽĆ molecular orbitals ’üĮ Bonding orbitals are occupied ’üĮ Anti-bonding orbitals are unoccupied ’üĮ The interaction of the two double bonds with each other to produce a delocalized system of pi electrons over all four atoms is known as conjugation. Dr.Samer HOUSHEH
- 12. Dr.Samer HOUSHEH
- 13. ’üĮ Chromophore: A covalently unsaturated group responsible for electronic absorption. or Any group of atoms that absorbs light whether or not a color is thereby produced. e.g. C=C, C=O, NO2 etc. ’üĮ A compound containing Chromophore is called chromogen. ’üĮ There are two types of Chromophore: ’āś Independent Chromophore: single Chromophore is sufficient to import color to the compound e.g. Azo group ’āś Dependent Chromophore: When more than one Chromophore is required to produce color. e.g. acetone having one ketone group is colorless where as diacetyl having two ketone group is yellow. Dr.Samer HOUSHEH
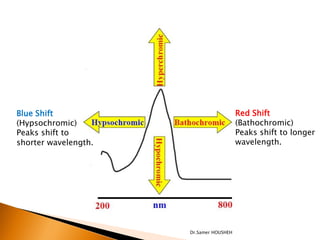
- 14. ’üĮ Auxochrome: A saturated group with non-bonding electron when attached to Chromophore alters both wavelengths as well as intensity of absorption. e.g. OH, NH2, NHR etc. ’üĮ Bathochromic group: The group which deepens the color of Chromophore is called bathochromic group. e.g. Primary, secondary and tertiary amino groups. ’üĮ Terminology: Auxochrome ’üĮ Bathochromic shift: (Red shift) shift of lambda max (╬╗max)to longer side or less energy is called bathochromic shift or read shift. This is due to substitution or solvent effect. ’üĮ Hypsochromic shift:(Blue shift)shift of lambda max (╬╗max)to shorter side and higher energy is called hypsochromic or blue shift. e.g solvent effect. ’üĮ Hyperchromic effect: an increase in absorption intensity ’üĮ Hypochromic effect: a decrease in absorption intensity Dr.Samer HOUSHEH
- 15. Blue Shift Red Shift (Hypsochromic) (Bathochromic) Peaks shift to Peaks shift to longer shorter wavelength. wavelength. Dr.Samer HOUSHEH
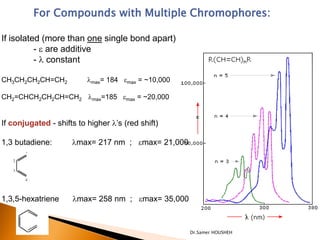
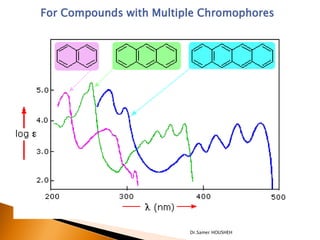
- 16. For Compounds with Multiple Chromophores: If isolated (more than one single bond apart) - e are additive - ’ü¼ constant CH3CH2CH2CH=CH2 ’ü¼max= 184 emax = ~10,000 CH2=CHCH2CH2CH=CH2 ’ü¼max=185 emax = ~20,000 If conjugated - shifts to higher ’ü¼ŌĆÖs (red shift) 1,3 butadiene: ’ü¼max= 217 nm ; emax= 21,000 1,3,5-hexatriene ’ü¼max= 258 nm ; emax= 35,000 Dr.Samer HOUSHEH
- 17. For Compounds with Multiple Chromophores Dr.Samer HOUSHEH
- 18. ’üĮ Different compounds may have very different absorption maxima and absorbances. ’üĮ Intensely absorbing compounds must be examined in dilute solution, so that significant light energy is received by the detector, and this requires the use of completely transparent(non-absorbing) solvents. ’üĮ Typical solvents are water, ethanol, hexane and cyclohexane. ’üĮ Solvents having double or triple bonds, or heavy atoms (e.g. S, Br & I) are generally avoided. ’üĮ Because the absorbance of a sample will be proportional to its molar concentration in the sample cuvette, a corrected absorption value known as the molar absorptivity is used when comparing the spectra of different compounds. Dr.Samer HOUSHEH
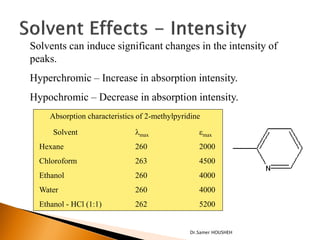
- 19. Solvents can induce significant changes in the intensity of peaks. Hyperchromic ŌĆō Increase in absorption intensity. Hypochromic ŌĆō Decrease in absorption intensity. Absorption characteristics of 2-methylpyridine Solvent ’ü¼max emax Hexane 260 2000 Chloroform 263 4500 Ethanol 260 4000 Water 260 4000 Ethanol - HCl (1:1) 262 5200 Dr.Samer HOUSHEH
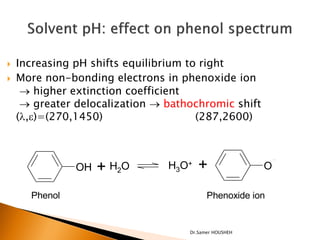
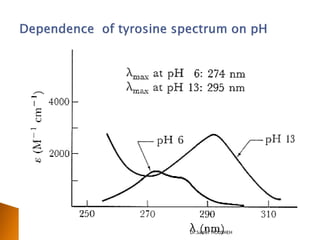
- 20. ’üĮ Increasing pH shifts equilibrium to right ’üĮ More non-bonding electrons in phenoxide ion ’é« higher extinction coefficient ’é« greater delocalization ’é« bathochromic shift (’ü¼,e)=(270,1450) (287,2600) OH + H2O H3O+ + O Phenol Phenoxide ion Dr.Samer HOUSHEH
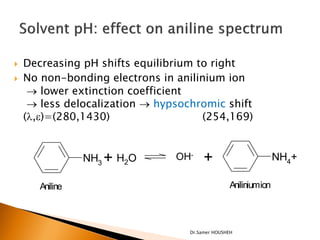
- 21. ’üĮ Decreasing pH shifts equilibrium to right ’üĮ No non-bonding electrons in anilinium ion ’é« lower extinction coefficient ’é« less delocalization ’é« hypsochromic shift (’ü¼,e)=(280,1430) (254,169) NH3 + H2O OH- + NH4+ Aniline Aniliniumion Dr.Samer HOUSHEH
- 22. Dr.Samer HOUSHEH
- 23. ’üĮ Scanning of UV Spectrum in different pH for some drugs ’é¢ Paracetamol (Acetaminophen) ’é¢ Caffeine Dr.Samer HOUSHEH
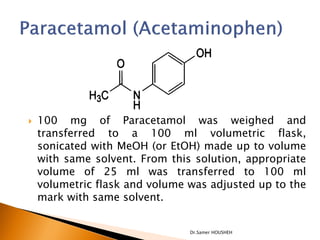
- 24. ’üĮ 100 mg of Paracetamol was weighed and transferred to a 100 ml volumetric flask, sonicated with MeOH (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with same solvent. Dr.Samer HOUSHEH
- 25. ’üĮ 100 mg of Paracetamol was weighed and transferred to a 100 ml volumetric flask, sonicated with MeOH (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with NaOH 0.1N. ’üĮ 100 mg of Paracetamol was weighed and transferred to a 100 ml volumetric flask, sonicated with MeOH (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with HCl 0.1N. Dr.Samer HOUSHEH
- 26. ’üĮ Make a scan for the three previous solutions in the UV spectroscopy and determine ╬╗max of the three solutions. ’üĮ Compare the three spectra and record your notes. ’üĮ Explain the presence or differences. Dr.Samer HOUSHEH
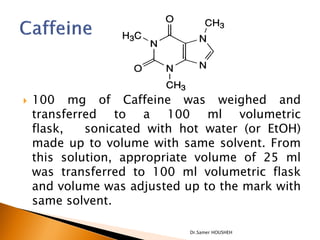
- 27. ’üĮ 100 mg of Caffeine was weighed and transferred to a 100 ml volumetric flask, sonicated with hot water (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with same solvent. Dr.Samer HOUSHEH
- 28. ’üĮ 100 mg of Aspirin was weighed and transferred to a 100 ml volumetric flask, sonicated with hot water (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with NaOH 0.1N. ’üĮ 100 mg of Aspirin was weighed and transferred to a 100 ml volumetric flask, sonicated with hot water (or EtOH) made up to volume with same solvent. From this solution, appropriate volume of 25 ml was transferred to 100 ml volumetric flask and volume was adjusted up to the mark with HCl 0.1N. Dr.Samer HOUSHEH
- 29. ’üĮ Make a scan for the three previous solutions in the UV spectroscopy and determine ╬╗max of the three solutions. ’üĮ Compare the three spectra and record your notes. ’üĮ Explain the presence or differences. Dr.Samer HOUSHEH
- 30. Quartz Cell Thanks for Paying Attention Dr.Samer HOUSHEH