Photo-Induced Modification of Starch
- 1. 1
- 2. 2
- 3. Starch ŌĆ£greenŌĆØ natural available biodegradable renewable low cost 3 ŌĆ£It is predicted that there will be a considerable demand in the near future for modified starchesŌĆØ
- 4. UV ŌĆ£eco- friendlyŌĆØ environment friendly economically viable fast safe not induce a significant increase in temperature no dependence on catalysts minimal sample preparation 4Bhat, R. and Karim, A.A. (2009). Impact of Radiation Processing on Starch, Comprehensive Reviews in Food Science and Food Safety, 8, 44-58.
- 11. 11 UV Radiation is defined as "that portion of the electromagnetic spectrum between x rays and visible light.ŌĆ£ UV Radiation, like all other electromagnetic radiation, is composed of energy particles called photons. The below image of the electromagnetic spectrum demonstrates the size and frequency of each energy. What is Ultraviolet Radiation?
- 12. 12 consequence of organic molecules absorbing a quantum of visible or UV light is to promote an electron to a vacant orbital of higher energy The energy of visible or UV light is of the same order as that of electronic transitions within atoms or molecules
- 13. 13 Once the molecule has absorbed this energy, it is said to be in an excited state from which various reaction possibilities arise. For an electron to be promoted from an energy level E1 to a higher energy level E2, the incident radiation must have a frequency which gives a value E greater than or equal to (E2-E1). Thus, ’üäE(E2-E1) = h’ü« The energy of the incident radiation is proportional to the frequency of the radiation E= h’ü«
- 14. Energy is re-emitted from the singlet state at a longer wavelength (S1 to S0 ŌĆ£flourescenceŌĆØ). Energy is re-emitted from the triplet state at an even longer wavelength (T1 to S0 ŌĆ£phosphorescenceŌĆØ). The singlet or triplet state undergoes a radiationless conversion to electronic ground state and gives off the energy in the form of heat (excited vibrational states). However, the molecule in its excited vibrational state can undergo a degradation reaction. Energy is transferred to another molecule which then dissipates the energy by the above three photophysical processes. 14 certain functional groups, such as a carbonyl group, can absorb the UV radiation and be excited to higher energy state. The excited electron usually returns ŌĆśunchangedŌĆÖ to the electronic ground state because the energy is dissipated by one of the following radiative and macroradiative photophysical routes:
- 15. ? Photosensitizer compound that absorbs light and transfers energy to a second compound and, in so doing, undergoes no net change. The second compound proceeds to produce radicals by photo cleavage or H- abstraction reactions. 15 UV light provides lower energy levels than other sources of ionizing radiation, it is impossible for direct cleavage of CŌĆōC or CŌĆōH bonds of starch molecules to occur for the formation of free radicals. Hence there is a need for a photosensitizer (photoinitiator) that can absorb a low-energy photon and become activated, leading to the formation of free radicals.
- 16. Common photosensitizers Benzoin and its derivatives benzophenone and its derivatives acetophenone and its derivatives 16
- 17. (I.S.C. = Intersystem crossing) Photoinitiation process
- 18. Under UV light excitation, the photoinitiator (I) is first promoted to its excited singlet state (1Ix). Then, via fast intersystem crossing, it converts into triplet state (3Ix). This transient state can undergo either direct cleavage of the molecule or hydrogen abstraction with amine compounds or hydrogen donors (DH). The triplet transient state can be quenched by the monomer by an energy transfer process that does not undergo any chain initiation. Accordingly, this process must be regarded as a dead loss pathway. The longer the life time of the triplet state, the more efficient the quenching process becomes. Thus, 18
- 19. BP2 H2O2
- 20. Effect of UV on starch properties Decrease in viscosity Increase in solubility increases water binding capacity 20
- 21. 21
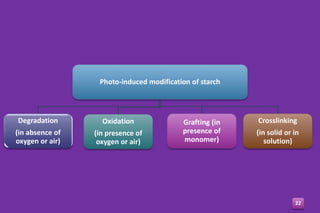
- 22. Photo-induced modification of starch Degradation (in absence of oxygen or air) Oxidation (in presence of oxygen or air) Grafting (in presence of monomer) Crosslinking (in solid or in solution) 22
- 23. All the energy coming from the Sun, from which the earth receives 1.5x1018 kWh per year, approximately 28000 times the consumption of all the world in that period. Solar radiation as source of light 23
- 24. Solar collectors No concentration or low temperature, up to 150o C Medium concentration or medium temperature, from 150o C to 400o C High concentration and high temperature, over 400o C. 24 what is important in photocatalysis is not only the amount of radiation collected, but its wavelength
- 25. Non-concentrating solar collectors for domestic heat water application 25
- 27. High concentrating solar collector 27
- 28. 28 Future work can be done on the basis of using photo initiators/UV system by establishing a ŌĆ£solar energy collectorŌĆØ to collect UV from sun light and use it instead of UV from the mercury lamp
- 29. 29 I am greatly thankful to Prof. James T. Guthrie Colour Chemistry Department, University of Leeds, England For gifting me the UV lamp and related accessories
- 30. Thank you 30