physical-properties-of-solution.pptxxxxx
- 3. What Is a Solution? ŌĆó is a type of homogeneous mixture that is made up of two or more substances. ŌĆó A homogeneous mixture is a type of mixture with a uniform composition.
- 4. Let's make use of our salt water example to talk about the two main parts of a solution. These are: Solute: this is the substance that makes up the minority of the solution, or this is the part that is dissolved. Example, the salt water, the solute is the salt. Solvent: this is the substance that makes up the majority of the solution. This is the part where the solute is dissolved. Example, the salt water, the solvent is water.
- 5. TYPES OF SOLUTIONS On the basis of physical states of solute and solvents Gaseous Solutions: gases can spontaneously mix in any proportion. Solid Solutions: they are generally called alloys, where two or more metals are present. Mercury alloys are called amalgams, and they can be liquid or solid. Liquid Solutions: liquid solutions are the most common and they can be obtained by dissolution of a gaseous, liquid or solid solute.
- 6. TYPES OF SOLUTIONS On the basis of dissolution of solute in solvent A saturated solution contains the maximum amount of a solute that will dissolve in a given solvent at a specific temperature. An unsaturated solution contains less solute than the solvent has the capacity to dissolve at a specific temperature. A supersaturated solution contains more solute than is present in a saturated solution at a specific temperature.
- 8. Energies of Solution Formation ŌĆó "Like dissolves like" Step 1: Seperating the solution into individual components the solute (expanding the solute). Step 2: Overcoming intermolecular forces in the solvent to make room for the solute (expanding the solute) Step 3: Allowing the solute and solvent to interact to form the solution ŌĆó Enthalpy of hydration is step 1 and step 2 combined into 1 step
- 9. Energies of Solution Formation
- 11. Concentration Units ’āś The concentration of a solution is the amount of solute present in a given quantity of solvent or solution.
- 12. a.) Percent by Mass % by mass = mass of solute mass of solute + mass of solvent x 100% = mass of solute solute mass of solution x 100% Percent by Volume % by volume = volume of solute volume of solution x 100%
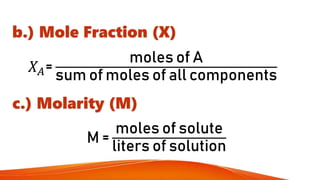
- 13. b.) Mole Fraction (X) ØæŗØÉ┤= moles of A sum of moles of all components c.) Molarity (M) M = moles of solute liters of solution
- 14. d.) Molality (m) m = moles of solute mass of solvent (kg) e.) Parts per million ppm = mass of solute mass of solution x 106
- 15. ppm ’āś1 ppm is one part by weight, or volume, of solute in 1 million parts by weight, or volume, of solution. ’āś In weight/volume (w/v) terms,1 ppm = 1g m-3 = 1 mg L-1 = 1 ╬╝g mL-1 ’āś In weight/weight (w/w) terms,1 ppm = 1 mg kg- 1 = 1 ╬╝g g-1
- 17. SOLUTION STOICHIOMETRY ’āś it deals with quantities in chemical reactions taking place in solutions.
- 18. How many moles of water form when 25.0 mls of 0.100 M HNO3 (nitric acid) solution is completely neutralized by NaOH (a base)? EXAMPLE
- 23. SOLUBILITY ’āś is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature.
- 24. Factors Affecting Solubility There are two direct factors that affect solubility: temperature and pressure. ŌĆó Temperature affects the solubility of both solids and gases. ŌĆó Surface area does not affect how much of a solute will be dissolved, but it is a factor in how quickly or slowly the substance will dissolve. ŌĆó But pressure only affects the solubility of gases.
- 25. The Effect of Temperature on Solubility ’āś Temperature has a direct effect on solubility. ’āśFor the majority of ionic solids, increasing the temperature increases how quickly the solution can be made. ’āś As the temperature increases, the particles of the solid move faster, which increases the chances that they will interact with more of the solvent particles. ’āś This results in increasing the rate at which a solution occurs.
- 26. The Effect of Pressure on Solubility ’āś The second factor, pressure, affects the solubility of a gas in a liquid but never of a solid dissolving in a liquid. ’āś When pressure is applied to a gas that is above the surface of a solvent, the gas will move into the solvent and occupy some of the spaces between the particles of the solvent. ’āśThis gas pressure factor is expressed in HenryŌĆÖs law. HenryŌĆÖs law states that, at a given temperature, the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the liquid.
- 27. The Effect of Surface Area on the Rate of Dissolving ’āś If we were to increase the surface area of a solid, then it would have been broken into smaller pieces. We would do this to increase how quickly the solute would dissolve in solution ’āśIf you were to dissolve sugar in water, a sugar cube will dissolve slower than an equal amount of tiny pieces of sugar crystals. The combined surface area of all of the sugar crystals have a much greater surface area than the one sugar cube and will have more contact with the water molecules. This allows the sugar crystals to dissolve much more quickly.
- 29. Colligative Properties of Nonelectrolytes and Electrolyte Solutions ’āś depend on the concentration of solute particles but not on their chemical identity. ’āś The concentration of solute particles depends on the amount of dissolved solute as well as on its ability to dissociate to ions in solution Colligative Properties
- 30. ŌĆó Weak electrolytes ŌĆō dissociate partially (weak acids and bases) ŌĆó Strong electrolytes ŌĆō dissociate completely (soluble salts, strong acids and bases) ŌĆó Nonelectrolytes ŌĆō do not dissociate (many organic compounds) ŌĆó Nonvolatile Nonelectrolyte Solutions ŌĆō No dissociation; no vapor pressure (glucose, sugar, ŌĆ”)
- 31. Vapor pressure lowering (╬öP) ŌĆó the vapor pressure of the solvent over the solution (Psolv) is always lower than the vapor pressure over the pure solvent (P┬░solv) at a given temperature Raoult╩╝s law
- 32. RaoultŌĆÖs Law ŌĆó the vapor pressure of the solvent over the solution is directly proportional to the mole fraction of the solvent ŌĆó Followed strictly at all concentrations only by ideal solutions
- 33. Boiling point elevation (╬öTb) and freezing point depression (╬öTf) ŌĆó The solution boils at a higher temperature compared to the pure solvent (the solution has lower vapor P so it needs higher T to boil) ŌĆó The solution freezes at a lower temperature compared to the pure solvent
- 34. Osmotic pressure (╬Ā) ŌĆó Osmosis ŌĆō the flow of solvent trough a semi permeable membrane from a less concentrated into a more concentrated solution ŌĆó Semipermeable membrane ŌĆō the solute particles canŌĆÖt pass through
- 35. ’āś The solvent tends to flow into the solution where the disorder is greater
- 36. ŌĆó ╬Ā is the hydrostatic pressure necessary to stop the net flow of solvent caused by osmosis ╬Ā = MRT ╬Ā = (nsolute/Vsoln)RT M ŌĆō molarity of solution R ŌĆō gas constant; T ŌĆō temperature in K
- 37. ŌĆó The equation is the equivalent of the ideal gas law (P = nRT/V) applied to solutions. ŌĆó ╬Ā is the pressure the solute would exert if it were an ideal gas occupying alone the volume of the solution.
- 38. ŌĆó Osmosis is essential for controlling the shape and size of biological cells and purifying blood through dialysis ŌĆó Reverse osmosis ŌĆō reversing the flow by applying external pressure (used to purify sea water)