Pitting Corrosion_Rahul
Download as PPTX, PDF10 likes8,974 views
Pitting corrosion is a localized form of corrosion that leads to the formation of small cavities or holes in the material. It occurs when small areas become active (anodic) while the surrounding areas remain passive (cathodic). This creates galvanic cells that drive the corrosion process. Pitting corrosion initiates at defects on the material surface and then propagates in an autocatalytic manner. It is most common in alloys protected by a passive film when exposed to environments containing chlorides, oxygen, and stagnant conditions. Proper material selection, surface finishing, controlling environmental factors like pH and chloride levels, and using protective coatings or cathodic protection can help prevent pitting corrosion.
1 of 7
Downloaded 317 times



Recommended
Intergranular Corrosion



Intergranular CorrosionGulfam Hussain
Ìý
Intergranular Corrosion
Causes and Mechanism Intergranular Corrosion Types Of Intergranular CorrosionPresentation ON EROSION CORROSION



Presentation ON EROSION CORROSIONRISHABH SHARMA
Ìý
Rishabh Sharma's presentation discusses erosion corrosion, which is an increase in corrosion caused by a high relative velocity between a corrosive environment and a metal surface. It involves both chemical corrosion and mechanical wear as corroded metal is removed. The mechanisms are not fully understood but involve turbulent flow, suspended solids, and gas/liquid interactions. Erosion corrosion is more severe for softer metals and in equipment exposed to high velocities, turbulence, and mass transfer. Examples include pipes, valves, pumps and turbine blades. The presentation covers factors like pH, velocity, material choice, and surface films that influence erosion corrosion rates and provides prevention methods like design changes, environment modifications, material selection, and coatings.Corrosion ppt part 1



Corrosion ppt part 1Swastika Das
Ìý
The document discusses corrosion of metals and its various types. It defines corrosion as the deterioration of metal due to chemical reactions with the environment. Corrosion occurs via oxidation and causes metal loss. The main factors influencing corrosion are the metal composition, environmental chemicals, temperature, and design. Corrosion can be uniform, galvanic, pitting, intergranular or stress-related. Electrochemical corrosion involves the formation of anodes and cathodes on a metal surface.Corrosion types & prevention



Corrosion types & preventionvishalbailur1
Ìý
The document discusses different types of corrosion that can occur in metals. It describes wet/aqueous corrosion which occurs in the presence of water and can include uniform, galvanic, crevice, pitting, and other forms of corrosion. It also discusses dry/high temperature corrosion which mainly includes oxidation and corrosion in sulfur environments. The document provides detailed explanations and examples of each type of corrosion.Rate of Corrosion And Types of Corrosion



Rate of Corrosion And Types of Corrosionrealistic_friend
Ìý
The document discusses different types of corrosion and how to calculate corrosion rates. It describes 10 common types of corrosion including general attack, localized pitting and crevice corrosion, galvanic corrosion, stress corrosion cracking, and high temperature corrosion. It also explains that corrosion rates depend on factors like weight loss, metal density, surface area, and time, and can be determined using electrochemical measurements and Faraday's law.Chapter 4: Corrosion testing
(shared using VisualBee)



Chapter 4: Corrosion testing
(shared using VisualBee)Nima_s70
Ìý
This chapter discusses corrosion testing methods and considerations. It describes 4 types of corrosion tests: 1) laboratory tests using small specimens, 2) pilot-plant tests that duplicate full-scale operations, 3) actual service tests in operating plants, and 4) field tests using exposed specimens. Key factors discussed include the materials tested, specimen size and shape, surface preparation, measurement techniques, exposure methods, and types of tests like boiling tests and sea water tests. The goal of corrosion testing is to evaluate materials, mechanisms, and environments in a controlled and reproducible manner.Stress corrosion cracking



Stress corrosion crackingonlinemetallurgy.com
Ìý
Stress corrosion cracking is the failure of a normally ductile metal caused by the combined effect of tensile stress and a corrosive environment. Three factors are required for stress corrosion cracking to occur: a susceptible material, a tensile stress (either applied or residual), and a corrosive environment. Stress corrosion cracking leads to the formation of cracks that propagate in the material over time and eventually result in sudden brittle fracture.Corrosion and its Control



Corrosion and its ControlDr. Arun Sharma
Ìý
This ppt explains basics of corrosion, its significance, Mechanism of electrochemical and chemical corrosion, Cathodic protection, Anodic protection, Sacrificial protection, Galvanization, Concentration Corrosion, Pitting Corrosion and also describe about the prevention and control of corrosion with respect to protective coatings and modification in design.selective leaching type corrosion



selective leaching type corrosionJAYESH PAREKH
Ìý
Selective leaching, also called de-alloying or de-metalification, refers to the selective removal of one element from an alloy by corrosion processes. A common example is the dezincification of brass, where zinc is selectively removed leaving a porous copper structure. There are three steps in the mechanism of dezincification: (1) dissolution of the entire alloy, (2) replating of the more noble metal (copper), and (3) leaching away of the active metal (zinc). Dezincification can occur uniformly or in localized plugs and is caused by water containing sulfur, carbon dioxide, and oxygen. Prevention methods include using less susceptible alloys, adding inhibitors like tinErosion Corrosion 



Erosion Corrosion Gulfam Hussain
Ìý
Erosion corrosion occurs when the rate of material deterioration increases due to the combined effects of corrosion and mechanical wear from fluid flow. It can occur in pipes, valves, pumps and other equipment exposed to flowing liquids or gases. The mechanism involves turbulent flow damaging protective surface films and exposing the bare metal to chemical attack. Common signs are grooves, holes and valleys in the direction of flow. Prevention methods include design modifications to reduce turbulence, removing abrasive particles from the fluid, protective coatings, cathodic protection, and using more corrosion resistant materials.Corrosion inhibitors.ppt



Corrosion inhibitors.pptSriCharitha6
Ìý
Corrosion inhibitors are substances that, when added in small amounts to an aqueous corrosive environment, decrease the corrosion of a metal. There are two types of inhibitors: anodic inhibitors form protective films on metal surfaces to reduce corrosion at anodes, while cathodic inhibitors either slow the diffusion of hydrogen ions or increase the overvoltage of hydrogen evolution to reduce corrosion at cathodes. Common examples of both types of inhibitors are listed.Metal corrosion and its prevention



Metal corrosion and its preventionDr. Sandip Thorat
Ìý
This document discusses metal corrosion and its prevention. It defines corrosion as the destruction or deterioration of solid metallic materials due to chemical or electrochemical attack from their environment. Corrosion is a major problem worldwide that impacts safety, health, and the environment. The document describes various types of corrosion including uniform corrosion, pitting corrosion, stress corrosion cracking, galvanic corrosion, and erosion corrosion. It also discusses factors that influence corrosion and various methods to prevent corrosion, such as using corrosion inhibitors, cathodic protection, coatings, and selecting corrosion-resistant materials.corrosion presentation



corrosion presentationakshaykhanna1997
Ìý
Virtually all engineering materials will corrode or decay over time when exposed to their environment. The rate of decay depends on the material and conditions. Like the human body, materials require protection from extreme temperatures, pressures, and harmful gases through coatings, inhibitors, alloys, maintenance and inspection. Corrosion causes the disintegration of materials into constituent atoms via chemical reactions with the surroundings like oxygen, and reduces material strength, lifetime and properties. Data on corrosion rates helps determine if a material is suitable for an application, with over 50 mils per year generally unsuitable. Common types of corrosion include uniform, galvanic, pitting, stress, erosion and microbial. Protections methods aim to control reactions or provide permanent barriersDifferent types of corrosion



Different types of corrosionsourabhrana21
Ìý
1. Electrochemical corrosion occurs via oxidation and reduction reactions when a metal is in contact with an electrolyte like an acid or salt solution. Electrons generated through oxidation must be consumed in reduction reactions.
2. There are several types of electrochemical corrosion including galvanic, pitting, differential aeration, water line, crevice, stress, and intragranular corrosion.
3. Galvanic corrosion occurs when two dissimilar metals are in electrical contact in a corrosive environment, leading to accelerated corrosion of the metal with the lower reduction potential. Common examples include zinc anodes on copper or steel structures.Forms of corrosion



Forms of corrosionProf. T. K. G. Namboodhiri
Ìý
A presentation giving the classification of corrosion and various forms of corrosion. Basic features of these various forms re given.
Cathodic and anodic protection



Cathodic and anodic protectionTHOMAS THANGADURAI K
Ìý
This document discusses cathodic and anodic protection techniques to prevent corrosion of metal structures. It describes two methods of cathodic protection: 1) sacrificial anodic protection which uses more reactive "sacrificial anodes" connected to the structure, and 2) impressed current cathodic protection which uses an external current source and inert anode. Applications include protecting underground pipelines, cables, ship hulls, tanks, and more. The document also covers anodic protection which makes the metal structure the anode and controls its potential to reduce corrosion, using a technique called potentiostat.Corrosion



Corrosionsankar n
Ìý
A brief introduction to corrosion and types of corrosion, such as pitting corrosion.
Cavitations corrosion
Galvanic corrosion.
Fretting corrosion.
Crevice corrosion.
Intergranular and transgranular corrosion,
Stress corrosion
(Pitting corrosion and crevice corrosion)



(Pitting corrosion and crevice corrosion)Mustafa Hasan
Ìý
This document discusses pitting corrosion and crevice corrosion in metals. It defines these types of localized corrosion and explains their mechanisms. Pitting corrosion occurs in localized holes in metals and is difficult to detect. Crevice corrosion occurs in cracks and crevices where conditions differ from the bulk solution, leading to acidification and accelerated corrosion. Both types of corrosion are influenced by parameters like chloride concentration, temperature, material properties, and coatings. The document provides diagrams illustrating the corrosion mechanisms and test methods for evaluating resistance to pitting and crevice corrosion.Types of corrosions



Types of corrosionsAmar Ilindra
Ìý
14 Types of Corrosion explained in an awesome manner
Update 26 June 2019: I have enabled the Download option and now everyone can download the "Types of corrosions" PPT and reuse the slides :) I wish I have done this earlier.
Follow my blogs at https://www.geekdashboard.com/ Corrosion Monitoring



Corrosion MonitoringDhanesh S
Ìý
This document discusses corrosion monitoring and crack monitoring techniques for condition monitoring of machines. It describes several common corrosion monitoring methods like weight loss, electrical resistance, linear polarization, and ultrasonic testing. It also discusses some crack monitoring methods like visual inspection and non-destructive testing techniques like penetrant testing, magnetic particle testing, and ultrasonic testing. The goal is to monitor deterioration rates and measure changes in crack width over time to assess structural integrity.Corrosion seminar 1



Corrosion seminar 1bikashgohain2
Ìý
Corrosion is the deterioration of a metal due to chemical reactions with its environment. There are two main types: dry corrosion which occurs without moisture via chemical reactions with gases, and wet corrosion which occurs in conducting liquids and involves electrochemical processes. Common forms of wet corrosion include uniform corrosion, galvanic corrosion, pitting corrosion, and stress corrosion. Corrosion can cause economic losses and reduce metal efficiency by decreasing production rates and increasing costs.Corrosion



CorrosionArun negemiya
Ìý
This document discusses various forms of corrosion that can occur in metals. It begins by defining corrosion and explaining the factors that influence it. It then describes several specific types of corrosion: general/uniform corrosion, galvanic corrosion, crevice corrosion, pitting corrosion, intergranular corrosion, dealloying, erosion corrosion, stress corrosion, hydrogen damage including hydrogen blistering and hydrogen embrittlement. For each type of corrosion, the document discusses the mechanism and provides methods for prevention.Corrosive Damage In Metals & Its Prevention



Corrosive Damage In Metals & Its PreventionProf. T. K. G. Namboodhiri
Ìý
Corrosion is the degradation of materials due to reaction with the environment. It affects metals, non-metals, and living tissues, causing damage like material loss and increased costs. Proper material selection, design modifications, environmental control, and protective coatings or cathodic protection can prevent a majority of corrosion damage and reduce annual economic losses estimated to be 3-5% of global GDP.Corrosion prevention



Corrosion preventionHarish Chopra
Ìý
This document discusses various methods for preventing corrosion of metals. It begins by introducing the importance of preventing corrosion, which causes huge economic losses. The main methods discussed are modifying the material through coatings or alloys to increase corrosion resistance, using corrosion inhibitors, cathodic protection, and protective coatings. For coatings, it describes metallic coatings like electroplating, electroless plating and zinc coatings, as well as inorganic coatings like anodized aluminum coatings. It also discusses factors that affect the corrosion rate like the metal's purity, environment pH, and presence of impurities.Loss of corrosion and prevention of corrosion



Loss of corrosion and prevention of corrosionAl Fahad
Ìý
we know a lot about loss of due to corrosion and prevention of corrosion. a lot of information you can know in this slideCorrosion in Metals 



Corrosion in Metals Mohammud Hanif Dewan M.Phil.
Ìý
Corrosion is the deterioration of metals through chemical reactions with the environment. There are several types of corrosion, including galvanic corrosion which occurs when two dissimilar metals are in electrical contact in an electrolyte, leading the less noble metal to corrode faster. Pitting corrosion causes localized holes or pits in the metal surface. Selective leaching corrosion removes specific elements from alloys, like removing zinc from brass. Proper material selection, coatings, inhibitors, and cathodic protection can prevent various corrosion types.Corrosion engineering



Corrosion engineeringArif Raihan
Ìý
Corrosion is the degradation or destruction of a metal due to a reaction with its environment. It occurs via either a chemical or electrochemical process. There are four requirements for electrochemical corrosion to occur: an anode, cathode, electrically conductive medium, and a metallic path connecting the anode and cathode. Corrosion can cause economic losses through damage to structures and equipment, reduce safety, and waste limited metal resources. It is important to study corrosion to prevent failures and catastrophic accidents while extending equipment lifetime in a cost-effective manner.Corrossion focus on polarization



Corrossion focus on polarizationsruthi Sudhakar
Ìý
Corrosion is the destructive attack of a metal by chemical or electrochemical reaction with its environment. There are three main types of polarization that influence the corrosion rate - concentration polarization, activation polarization, and IR drop. Concentration polarization occurs when the rate of the electrochemical reaction is controlled by the mass transport of reactants or products. Activation polarization is caused by an energy barrier that must be overcome for the electrochemical reaction to proceed. IR drop is the potential drop across the electrolyte due to its resistance. The total polarization is the sum of the individual polarization contributions. Cathodic polarization generally decreases the corrosion rate while anodic polarization can either increase or decrease the corrosion rate depending on whether the metal is in an active or passive state.Corrision word doc.



Corrision word doc.Umer Farooq
Ìý
Corrosion is the process by which metals return to their natural oxidation states through redox reactions, often with oxygen. It causes deterioration of metal products and structures. There are three main components required for corrosion: a metal, oxygen, and an electrolyte like water. Corrosion occurs through either generalized thinning of the metal surface or localized pitting. It is an electrochemical process where the metal acts as an anode and is oxidized while oxygen is reduced at the cathode. Corrosion can be prevented using methods like painting, sacrificial anodes, cathodic protection, or exploiting natural corrosion products that form protective barriers.Chapter5 150109005402-conversion-gate02



Chapter5 150109005402-conversion-gate02Cleophas Rwemera
Ìý
Corrosion occurs via electrochemical reactions between a material, usually a metal, and its environment. There are several types of corrosion including uniform corrosion, pitting, crevice corrosion, and intergranular corrosion. Corrosion can be prevented through methods like cathodic protection, selecting corrosion-resistant materials, using protective coatings, designing to avoid corrosion-prone situations, and alloying metals to enhance corrosion resistance. Managing corrosion is important as it can lead to infrastructure and equipment failures which are costly to repair and can impact safety.More Related Content
What's hot (20)
selective leaching type corrosion



selective leaching type corrosionJAYESH PAREKH
Ìý
Selective leaching, also called de-alloying or de-metalification, refers to the selective removal of one element from an alloy by corrosion processes. A common example is the dezincification of brass, where zinc is selectively removed leaving a porous copper structure. There are three steps in the mechanism of dezincification: (1) dissolution of the entire alloy, (2) replating of the more noble metal (copper), and (3) leaching away of the active metal (zinc). Dezincification can occur uniformly or in localized plugs and is caused by water containing sulfur, carbon dioxide, and oxygen. Prevention methods include using less susceptible alloys, adding inhibitors like tinErosion Corrosion 



Erosion Corrosion Gulfam Hussain
Ìý
Erosion corrosion occurs when the rate of material deterioration increases due to the combined effects of corrosion and mechanical wear from fluid flow. It can occur in pipes, valves, pumps and other equipment exposed to flowing liquids or gases. The mechanism involves turbulent flow damaging protective surface films and exposing the bare metal to chemical attack. Common signs are grooves, holes and valleys in the direction of flow. Prevention methods include design modifications to reduce turbulence, removing abrasive particles from the fluid, protective coatings, cathodic protection, and using more corrosion resistant materials.Corrosion inhibitors.ppt



Corrosion inhibitors.pptSriCharitha6
Ìý
Corrosion inhibitors are substances that, when added in small amounts to an aqueous corrosive environment, decrease the corrosion of a metal. There are two types of inhibitors: anodic inhibitors form protective films on metal surfaces to reduce corrosion at anodes, while cathodic inhibitors either slow the diffusion of hydrogen ions or increase the overvoltage of hydrogen evolution to reduce corrosion at cathodes. Common examples of both types of inhibitors are listed.Metal corrosion and its prevention



Metal corrosion and its preventionDr. Sandip Thorat
Ìý
This document discusses metal corrosion and its prevention. It defines corrosion as the destruction or deterioration of solid metallic materials due to chemical or electrochemical attack from their environment. Corrosion is a major problem worldwide that impacts safety, health, and the environment. The document describes various types of corrosion including uniform corrosion, pitting corrosion, stress corrosion cracking, galvanic corrosion, and erosion corrosion. It also discusses factors that influence corrosion and various methods to prevent corrosion, such as using corrosion inhibitors, cathodic protection, coatings, and selecting corrosion-resistant materials.corrosion presentation



corrosion presentationakshaykhanna1997
Ìý
Virtually all engineering materials will corrode or decay over time when exposed to their environment. The rate of decay depends on the material and conditions. Like the human body, materials require protection from extreme temperatures, pressures, and harmful gases through coatings, inhibitors, alloys, maintenance and inspection. Corrosion causes the disintegration of materials into constituent atoms via chemical reactions with the surroundings like oxygen, and reduces material strength, lifetime and properties. Data on corrosion rates helps determine if a material is suitable for an application, with over 50 mils per year generally unsuitable. Common types of corrosion include uniform, galvanic, pitting, stress, erosion and microbial. Protections methods aim to control reactions or provide permanent barriersDifferent types of corrosion



Different types of corrosionsourabhrana21
Ìý
1. Electrochemical corrosion occurs via oxidation and reduction reactions when a metal is in contact with an electrolyte like an acid or salt solution. Electrons generated through oxidation must be consumed in reduction reactions.
2. There are several types of electrochemical corrosion including galvanic, pitting, differential aeration, water line, crevice, stress, and intragranular corrosion.
3. Galvanic corrosion occurs when two dissimilar metals are in electrical contact in a corrosive environment, leading to accelerated corrosion of the metal with the lower reduction potential. Common examples include zinc anodes on copper or steel structures.Forms of corrosion



Forms of corrosionProf. T. K. G. Namboodhiri
Ìý
A presentation giving the classification of corrosion and various forms of corrosion. Basic features of these various forms re given.
Cathodic and anodic protection



Cathodic and anodic protectionTHOMAS THANGADURAI K
Ìý
This document discusses cathodic and anodic protection techniques to prevent corrosion of metal structures. It describes two methods of cathodic protection: 1) sacrificial anodic protection which uses more reactive "sacrificial anodes" connected to the structure, and 2) impressed current cathodic protection which uses an external current source and inert anode. Applications include protecting underground pipelines, cables, ship hulls, tanks, and more. The document also covers anodic protection which makes the metal structure the anode and controls its potential to reduce corrosion, using a technique called potentiostat.Corrosion



Corrosionsankar n
Ìý
A brief introduction to corrosion and types of corrosion, such as pitting corrosion.
Cavitations corrosion
Galvanic corrosion.
Fretting corrosion.
Crevice corrosion.
Intergranular and transgranular corrosion,
Stress corrosion
(Pitting corrosion and crevice corrosion)



(Pitting corrosion and crevice corrosion)Mustafa Hasan
Ìý
This document discusses pitting corrosion and crevice corrosion in metals. It defines these types of localized corrosion and explains their mechanisms. Pitting corrosion occurs in localized holes in metals and is difficult to detect. Crevice corrosion occurs in cracks and crevices where conditions differ from the bulk solution, leading to acidification and accelerated corrosion. Both types of corrosion are influenced by parameters like chloride concentration, temperature, material properties, and coatings. The document provides diagrams illustrating the corrosion mechanisms and test methods for evaluating resistance to pitting and crevice corrosion.Types of corrosions



Types of corrosionsAmar Ilindra
Ìý
14 Types of Corrosion explained in an awesome manner
Update 26 June 2019: I have enabled the Download option and now everyone can download the "Types of corrosions" PPT and reuse the slides :) I wish I have done this earlier.
Follow my blogs at https://www.geekdashboard.com/ Corrosion Monitoring



Corrosion MonitoringDhanesh S
Ìý
This document discusses corrosion monitoring and crack monitoring techniques for condition monitoring of machines. It describes several common corrosion monitoring methods like weight loss, electrical resistance, linear polarization, and ultrasonic testing. It also discusses some crack monitoring methods like visual inspection and non-destructive testing techniques like penetrant testing, magnetic particle testing, and ultrasonic testing. The goal is to monitor deterioration rates and measure changes in crack width over time to assess structural integrity.Corrosion seminar 1



Corrosion seminar 1bikashgohain2
Ìý
Corrosion is the deterioration of a metal due to chemical reactions with its environment. There are two main types: dry corrosion which occurs without moisture via chemical reactions with gases, and wet corrosion which occurs in conducting liquids and involves electrochemical processes. Common forms of wet corrosion include uniform corrosion, galvanic corrosion, pitting corrosion, and stress corrosion. Corrosion can cause economic losses and reduce metal efficiency by decreasing production rates and increasing costs.Corrosion



CorrosionArun negemiya
Ìý
This document discusses various forms of corrosion that can occur in metals. It begins by defining corrosion and explaining the factors that influence it. It then describes several specific types of corrosion: general/uniform corrosion, galvanic corrosion, crevice corrosion, pitting corrosion, intergranular corrosion, dealloying, erosion corrosion, stress corrosion, hydrogen damage including hydrogen blistering and hydrogen embrittlement. For each type of corrosion, the document discusses the mechanism and provides methods for prevention.Corrosive Damage In Metals & Its Prevention



Corrosive Damage In Metals & Its PreventionProf. T. K. G. Namboodhiri
Ìý
Corrosion is the degradation of materials due to reaction with the environment. It affects metals, non-metals, and living tissues, causing damage like material loss and increased costs. Proper material selection, design modifications, environmental control, and protective coatings or cathodic protection can prevent a majority of corrosion damage and reduce annual economic losses estimated to be 3-5% of global GDP.Corrosion prevention



Corrosion preventionHarish Chopra
Ìý
This document discusses various methods for preventing corrosion of metals. It begins by introducing the importance of preventing corrosion, which causes huge economic losses. The main methods discussed are modifying the material through coatings or alloys to increase corrosion resistance, using corrosion inhibitors, cathodic protection, and protective coatings. For coatings, it describes metallic coatings like electroplating, electroless plating and zinc coatings, as well as inorganic coatings like anodized aluminum coatings. It also discusses factors that affect the corrosion rate like the metal's purity, environment pH, and presence of impurities.Loss of corrosion and prevention of corrosion



Loss of corrosion and prevention of corrosionAl Fahad
Ìý
we know a lot about loss of due to corrosion and prevention of corrosion. a lot of information you can know in this slideCorrosion in Metals 



Corrosion in Metals Mohammud Hanif Dewan M.Phil.
Ìý
Corrosion is the deterioration of metals through chemical reactions with the environment. There are several types of corrosion, including galvanic corrosion which occurs when two dissimilar metals are in electrical contact in an electrolyte, leading the less noble metal to corrode faster. Pitting corrosion causes localized holes or pits in the metal surface. Selective leaching corrosion removes specific elements from alloys, like removing zinc from brass. Proper material selection, coatings, inhibitors, and cathodic protection can prevent various corrosion types.Corrosion engineering



Corrosion engineeringArif Raihan
Ìý
Corrosion is the degradation or destruction of a metal due to a reaction with its environment. It occurs via either a chemical or electrochemical process. There are four requirements for electrochemical corrosion to occur: an anode, cathode, electrically conductive medium, and a metallic path connecting the anode and cathode. Corrosion can cause economic losses through damage to structures and equipment, reduce safety, and waste limited metal resources. It is important to study corrosion to prevent failures and catastrophic accidents while extending equipment lifetime in a cost-effective manner.Corrossion focus on polarization



Corrossion focus on polarizationsruthi Sudhakar
Ìý
Corrosion is the destructive attack of a metal by chemical or electrochemical reaction with its environment. There are three main types of polarization that influence the corrosion rate - concentration polarization, activation polarization, and IR drop. Concentration polarization occurs when the rate of the electrochemical reaction is controlled by the mass transport of reactants or products. Activation polarization is caused by an energy barrier that must be overcome for the electrochemical reaction to proceed. IR drop is the potential drop across the electrolyte due to its resistance. The total polarization is the sum of the individual polarization contributions. Cathodic polarization generally decreases the corrosion rate while anodic polarization can either increase or decrease the corrosion rate depending on whether the metal is in an active or passive state.Similar to Pitting Corrosion_Rahul (20)
Corrision word doc.



Corrision word doc.Umer Farooq
Ìý
Corrosion is the process by which metals return to their natural oxidation states through redox reactions, often with oxygen. It causes deterioration of metal products and structures. There are three main components required for corrosion: a metal, oxygen, and an electrolyte like water. Corrosion occurs through either generalized thinning of the metal surface or localized pitting. It is an electrochemical process where the metal acts as an anode and is oxidized while oxygen is reduced at the cathode. Corrosion can be prevented using methods like painting, sacrificial anodes, cathodic protection, or exploiting natural corrosion products that form protective barriers.Chapter5 150109005402-conversion-gate02



Chapter5 150109005402-conversion-gate02Cleophas Rwemera
Ìý
Corrosion occurs via electrochemical reactions between a material, usually a metal, and its environment. There are several types of corrosion including uniform corrosion, pitting, crevice corrosion, and intergranular corrosion. Corrosion can be prevented through methods like cathodic protection, selecting corrosion-resistant materials, using protective coatings, designing to avoid corrosion-prone situations, and alloying metals to enhance corrosion resistance. Managing corrosion is important as it can lead to infrastructure and equipment failures which are costly to repair and can impact safety.Chapter 5: Corrosion & Non-ferrous Metal



Chapter 5: Corrosion & Non-ferrous Metalsyar 2604
Ìý
This topic describes two main categories of corrosion. It also explains the electrochemical corrosion phenomena and the differences between the types of corrosion. This topic also states the corrosion preventive steps.Corrosion and Environmental Degradation of Materials-2.pdf



Corrosion and Environmental Degradation of Materials-2.pdfponjustin1
Ìý
The document discusses corrosion and passivity of metals and alloys. It describes how alloying elements like chromium and nickel can reduce the critical potential of iron, allowing it to form a passive film. It also discusses how increasing the noble metal content of alloys can reduce both the critical potential and passive potential. The document covers several factors that influence corrosion rates, such as temperature, oxidizer concentration, and cathodic reactions. It provides examples of localized corrosion like crevice corrosion and describes the conditions under which it typically occurs.Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))



Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))Afzal Imam
Ìý
Corrosion is a process that occurs and encountered by us in our everyday lives. This presentation gives a basic idea about the process of corrosion. However, it is not in any way similar to the textbook knowledge provided by our schools and curriculum during high school years. This is designed to explain and clarify the concepts of corrosion to its viewers.
This presentation focuses on the topic of "Corrosion" in the subject basic chemistry usually taught in 1st or 2nd semester during B.Tech / B.E. courses. Other than the fact that this presentation is detailed oriented, this document can also be viewed for general information purposes as it is designed and explained in the simplest terms possible. Attractive and illustrative images are also used for the user to be able easily and quickly grasp the concept.corrosion.pdf..It is in the form of ppt.



corrosion.pdf..It is in the form of ppt.BhoomikaAdarshe
Ìý
This is the notes of corrosion..Which is useful to study the pharmaceutical Engineering subject.Theories_of_corrosion_and_its types.pptx



Theories_of_corrosion_and_its types.pptxrushikeshkaldate
Ìý
Theories of corrosion its mechanism with typesCorrosion and degradation of materials



Corrosion and degradation of materialsGhassan Alshahiri
Ìý
This document defines corrosion as the deterioration of a material due to reaction with its environment, especially oxygen. Corrosion is an electrochemical process that occurs when a metal is exposed to oxygen and an electrolyte like water. Three factors are required for corrosion: a metal, oxygen, and an electrolyte. Corrosion causes deterioration of manufactured products and infrastructure. Understanding and preventing corrosion is important for maintaining machinery and structures. Corrosion occurs through oxidation and reduction reactions and can be localized or generalized. Methods to prevent corrosion include painting, sacrificial anodes, cathodic protection, and passivation.Unit 2-science-of-corrosion



Unit 2-science-of-corrosionanuragmbst
Ìý
1. Corrosion is the deterioration and loss of solid metallic material by chemical or electrochemical attack by its environment.
2. There are two main types of corrosion: dry/chemical corrosion which occurs through direct chemical action, and wet/electrochemical corrosion which occurs when a conducting liquid is in contact with the metal.
3. Wet corrosion occurs via separate anodic and cathodic reactions - the anodic reaction involves metal dissolution or compound formation, while the cathodic reaction involves hydrogen evolution in acidic environments or oxygen absorption in basic environments.CORROSION ENGINEERING.pptx



CORROSION ENGINEERING.pptxBharatKumarHumagai
Ìý
This document discusses corrosion engineering and provides details on various corrosion topics. It begins with an introduction to corrosion and defines it as the deterioration of metal through chemical or electrochemical reactions with the environment. Some key points covered include:
- Corrosion costs the US economy $300 billion per year. Common examples of corrosion are rusting of iron when exposed to air and the formation of a green or blue film on copper in moist air.
- An electrochemical cell converts chemical energy of an indirect redox reaction into electrical energy. During corrosion, the metal being corroded acts as the anode and loses electrons/dissolves while another metal acts as the cathode and gains electrons.
- The main types of corrosionChapter 5



Chapter 5Mohd Nurilhadi Darmi
Ìý
Corrosion is the destruction of metals through reaction with the environment. It can occur in dry or wet environments and causes economic and safety issues. There are two main types of corrosion: general/uniform corrosion, which occurs at the same rate over the entire surface, and localized corrosion, which affects only certain areas. Methods of preventing corrosion include proper material selection, protective coatings like paint and plating, cathodic protection, and design considerations. Non-ferrous metals are metals that do not contain much iron and include aluminum, copper, zinc, and others which are used due to properties like corrosion resistance.vnd.openxmlformats-officedocument.presentationml.presentation&rendition=1.pptx



vnd.openxmlformats-officedocument.presentationml.presentation&rendition=1.pptxVictus4
Ìý
The document discusses corrosion and its causes. Corrosion occurs via chemical or electrochemical reactions between a metal and its environment that cause deterioration. It can be caused by oxygen, hydrogen, electrical currents, stress, or bacteria. Corrosion occurs via dry/chemical reactions directly with gases or wet/electrochemical reactions in an electrolyte that form anodes and cathodes. The rate depends on factors like the metal's position in the galvanic series and properties of any surface oxide or corrosion product layer.Corrosion



CorrosionGas & Oil Pakistan Ltd.
Ìý
Group 6 presents information on corrosion, including definitions, types of corrosion, factors that affect corrosion rates, and methods for preventing corrosion. The group members are Waqas Ahmad, Umair Aslam, Tayyab Naveed, Muhammad Umair, and Muhammad Mudeser khalid. Corrosion is defined as the decay of metal due to chemical reactions with gases in the atmosphere. There are two main types of corrosion: dry corrosion caused by direct chemical reactions, and wet corrosion which is an electrochemical process. Factors that influence corrosion rates include the metal's position in the galvanic series, purity, physical state, and properties of the corrodent environment. Prevention methods consist of modifyingForms of corrosion



Forms of corrosionchemnidhi
Ìý
There are several types of corrosion that can occur:
1. Uniform and galvanic corrosion which results from direct chemical attack or from dissimilar metals in contact being exposed to an electrolyte.
2. Erosion corrosion caused by abrasive fluid flow removing a metal's protective surface film.
3. Crevice and pitting corrosion which are localized forms of corrosion occurring in cracks, crevices or defects in a metal surface.
4. Intergranular corrosion which attacks grain boundaries in metals or alloys.
5. Other forms include exfoliation, selective leaching, stress corrosion cracking, waterline corrosion affecting ship hulls, soil corrosion depending on soil conditions, and microbiologically influenced corrosion caused byCorrosion.pptx



Corrosion.pptxSneha10D
Ìý
Corrosion is an electrochemical process where a metal oxidizes and dissolves into its environment. There are several types of corrosion including uniform corrosion, galvanic corrosion, pitting corrosion, and crevice corrosion. Uniform corrosion proceeds uniformly over the entire metal surface. Galvanic corrosion occurs when two dissimilar metals are electrically coupled in a corrosive electrolyte. Pitting and crevice corrosion are localized forms of attack that can cause perforation. Cathodic protection is a technique to control the corrosion of a metal by making it the cathode of an electrochemical cell.Corrosion 



Corrosion Dipal Prajapati
Ìý
This document discusses various types and theories of corrosion. It begins by introducing corrosion as the chemical reaction between a metal and its environment that causes the metal to deteriorate. It then describes three main theories of corrosion: the acid theory, dry/chemical theory, and galvanic/electrochemical theory. The rest of the document details eight specific types of corrosion including uniform, pitting, intergranular, exfoliation, stress, crevice, galvanic, and erosion corrosion. It provides examples and explanations for each type.I/II SEM BE, VTU, ENGINEERING CHEMISTRY , Module 2



I/II SEM BE, VTU, ENGINEERING CHEMISTRY , Module 2rashmi m rashmi
Ìý
1. The document discusses various types of corrosion including dry corrosion, wet corrosion, differential metal corrosion, differential aeration corrosion, pitting corrosion, stress corrosion, and water line corrosion.
2. It explains the electrochemical theory of corrosion and factors that affect the rate of corrosion such as the nature of the metal, corrosion product, potential difference, anodic/cathodic areas, pH, temperature, and conductivity.
3. Methods of corrosion control discussed are anodizing, phosphating, galvanization, and tinning which involve coating metals with protective layers to prevent corrosion. Anodizing forms a protective aluminum oxide layer while galvanization coats iron with zinc and tinning coats iron with tin.Factors affecting and prevention_Corrosion (1).ppt



Factors affecting and prevention_Corrosion (1).pptToranSahu18
Ìý
Corrosion Corrosion bahut jyada corrosion Corrosion engineering



Corrosion engineeringAtul Shinde
Ìý
Corrosion is the spontaneous reaction between a material like steel and its environment that degrades the material over time. For ships, corrosion poses a major problem as it can compromise the structural integrity of the vessel. There are two main methods to prevent corrosion - cathodic protection, which makes the structure negative to corrosion, and coatings, which act as a barrier between the steel and environment. Effective coatings must adhere well to the steel, be impermeable to water and oxygen, and have a thickness and pigmentation that limits penetration over the life of the coating.Pitting Corrosion_Rahul
- 1. Presentation on Pitting Corrosion Prepared by- RAHUL DEO SONAR Jr. Metallurgist 1 Metallurgy Dept.- Dammam Lab-KSA
- 2. INDEX Definition of Pitting Corrosion Mechanism Common Environments of Pitting Corrosion Prevention 2
- 3. Definition It is a form of entirely localized corrosion. Leads to formation of cavities or holes in the material. Considered to be the most dangerous and insidious type of corrosion. Rate of corrosion is higher than uniform corrosion because of large cathode/anode ratio. Difficult to detect and measure. Its is autocatalytic in nature. Occurs usually in the direction of gravity. Fig.1: Pitting corrosion on a Stainless Steel surface 3
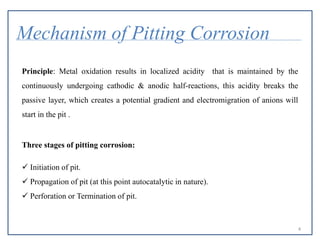
- 4. Mechanism of Pitting Corrosion Principle: Metal oxidation results in localized acidity that is maintained by the continuously undergoing cathodic & anodic half-reactions, this acidity breaks the passive layer, which creates a potential gradient and electromigration of anions will start in the pit . Three stages of pitting corrosion:  Initiation of pit.  Propagation of pit (at this point autocatalytic in nature).  Perforation or Termination of pit. 4
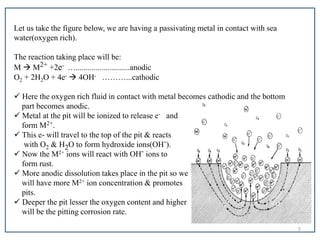
- 5. Let us take the figure below, we are having a passivating metal in contact with sea water(oxygen rich). The reaction taking place will be: M ïƒ M2+ +2e- …...........................anodic O2 + 2H2O + 4e- ïƒ 4OH- ………...cathodic  Here the oxygen rich fluid in contact with metal becomes cathodic and the bottom part becomes anodic.  Metal at the pit will be ionized to release e- and form M2+.  This e- will travel to the top of the pit & reacts with O2 & H2O to form hydroxide ions(OH-).  Now the M2+ ions will react with OH- ions to form rust.  More anodic dissolution takes place in the pit so we will have more M2+ ion concentration & promotes pits.  Deeper the pit lesser the oxygen content and higher will be the pitting corrosion rate. 5
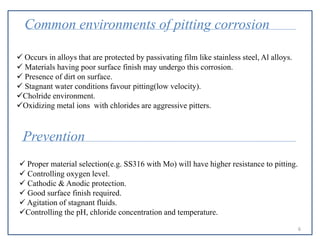
- 6. Common environments of pitting corrosion  Occurs in alloys that are protected by passivating film like stainless steel, Al alloys.  Materials having poor surface finish may undergo this corrosion.  Presence of dirt on surface.  Stagnant water conditions favour pitting(low velocity). Cholride environment. Oxidizing metal ions with chlorides are aggressive pitters. Prevention  Proper material selection(e.g. SS316 with Mo) will have higher resistance to pitting.  Controlling oxygen level.  Cathodic & Anodic protection.  Good surface finish required.  Agitation of stagnant fluids. Controlling the pH, chloride concentration and temperature. 6
- 7. 7

