Pre Clinical Studies
- 2. Debashish Sarkar Institute of Pharmacy- Nirma University 14MPH802
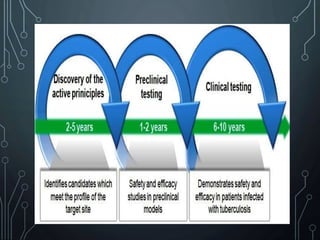
- 3. PRECLINICAL TRIALS âĒ Preclinical trials or non clinical trials â are laboratory test of a new drug substance or medical devices, usually done on animal subjects, to see whether the treatment really works and if it is safe to test on humans. âĒ The main goals of pre-clinical studies are to determine a product's ultimate safety profile. âĒ Products may include new medical devices, drugs, gene therapy solutions, etc.
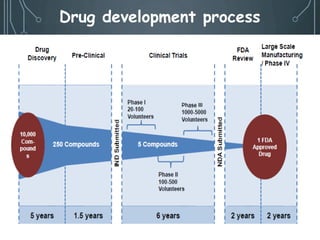
- 4. âĒ After identifying a compound, it is tested on animals to expose the whole pharmacological profile. âĒ Experiments are generally performed on rodent like mouse, rat, guinea pig, hamster, rabbit. âĒ After successful result, experiments are performed on larger animals like cat, dog, monkey. âĒ As the evaluation progresses unfavorable compounds get rejected at each step. âĒ So, that only few out of thousands reach the stage when administration to man is considered.
- 7. THERE ARE SEVERAL STEPS INVOLVED WITH DOING A PRE-CLINICAL TRIAL: File for approval as an Investigational New Drug 5 (IND) 4 Establish Effective and Toxic Doses 3 Screen the Drug in the Assay 2 Develop a Bioassay 1 Indentify a Drug Target
- 8. OBJECTIVES OF PRECLINICAL STUDIES âĒ The purpose of pre-clinical study is to develop adequate data to decide that it is reasonably safe to proceed with human trials of the drug. âĒ Means, a laboratory test of a new drug or a new medical device, usually done on animal subjects, to see if the treatment really works and if it is safe to test on humans âĒ However the main objective is to collect the data to submit to the FDA for IND filing.
- 9. THE TYPES OF STUDIES INCLUDED IN PRECLINICAL TRIALS 1.Screening Test 2. Tests on isolated organs, bacterial cultures 3. Tests on animal models of human disease 4. General observational test 5. Confirmatory tests and analogous activities 6. Mechanism of action 7. Systemic pharmacology 8. Quantitative test 9. Pharmacokinetics 10. Toxicity test
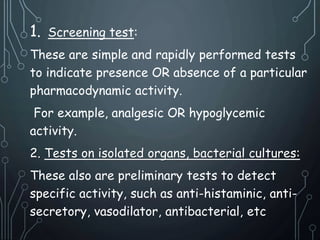
- 10. 1. Screening test: These are simple and rapidly performed tests to indicate presence OR absence of a particular pharmacodynamic activity. For example, analgesic OR hypoglycemic activity. 2. Tests on isolated organs, bacterial cultures: These also are preliminary tests to detect specific activity, such as anti-histaminic, anti-secretory, vasodilator, antibacterial, etc
- 11. 3. Tests on animal models of human disease: Animal models used such as kindled seizures in rats, genetically hypersensitive rats, experimental tuberculosis in mouse, etc. 4. General observational test: Drug is injected in tripling doses to small groups of mice which are observed for overt (hidden) effects. Preliminary clues are drawn from the profile of effect observed.
- 12. 5.Confirmatory tests and analogous activities: Compounds found active are taken up for detailed study by more elaborate (Complex) tests which confirm and characterize the activity. Other related activities also measured, like antipyretic and anti-inflammatory activity in an analgesic. 6. Mechanism of action: Attempts are made to find out the mechanism of action. E.g. whether an anti-hypertensive is an Îą blocker/Îē blocker/ ACE inhibitor/ calcium channel blocker, etc.
- 13. 7. Systemic pharmacology: âĒ Irrespective of the primary action of the drug, its effect on major organ systems such as nervous, cardio-vascular, respiratory, renal are worked out. 8. Quantitative test: The dose-response relationship, maximal effects and comparative efficacy with existing drug is carried out.
- 14. âĒ 9. Pharmacokinetics: âĒ The dose-response relationship, maximal effects and comparative efficacy with existing drug is carried out. âĒ 10. Toxicity test: Acute toxicity: âĒ Single high doses are given to small groups of animals that are observed for overt (hidden) effects and mortality for 1-3 days. âĒ The dose which kills 50% animals is called as LD50. âĒ Organ toxicity is examined by histopathology on all animals.
- 15. âĒ These whole tests are carried out under standardize procedure under âGood Laboratory Practiceâ (GLP). âĒ GLP applies to non-clinical studies conducted for the assessment of the safety or efficacy of chemicals (including pharmaceuticals) to man, animals and the environment
- 16. âĒ GLP specifically refers to a quality system of research laboratories and organizations to try to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of chemical (including pharmaceuticals) non-clinical safety tests; from physicochemical properties through acute to chronic toxicity tests. âĒ The original GLP regulatory mandate was promulgated in 1978 by US-FDA and published in the Federal Register 43 FR 59985-60020.
- 17. 21 CFR 312.38 âĒ Before the human studies can begin, an IND must be submitted to the Agency containing, among other things, information on any risks anticipated based on the results of pharmacologic and toxicological data collected during studies of the drug in animals (21 CFR 312.23(a)(8)). These basic safety tests are most often performed in rats and dogs. âĒ Pharmacology and toxicology information: Adequate information about the pharmacological and toxicological studies of the drug involving laboratory animals, or in vitro on the basis of which the the sponsor has concluded that it is reasonably safe to conduct the proposed clinical investigations.
- 18. Guidance documents are available from FDA that describe ways in which these requirements may be met. Such information is required to include the identification and qualifications of the individuals who evaluated the results of such studies and concluded that it is reasonably safe to begin the proposed investigations and a statement of where the investigations were conducted and where the records are available for inspection. As drug development proceeds, the sponsor is required to submit informational amendments, as appropriate, with additional information pertinent to safety.
- 19. 1.PHARMACOLOGY AND DRUG DISPOSITION. âĒ A section describing the pharmacological effects and mechanism(s) of action of the drug in animals, and information on the absorption, distribution, metabolism, and excretion of the drug, if known.
- 20. 2.TOXICOLOGY (a ) An integrated summary of the toxicological effects of the drug in animals and in vitro. Depending on the nature of the drug and the phase of the investigation, the description is to include the results of acute, subacute, and chronic toxicity tests; tests of the drug's effects on reproduction and the developing fetus; any special toxicity test related to the drug's particular mode of administration or conditions of use (e.g., inhalation, dermal, or ocular toxicology); and any in vitro studies intended to evaluate drug toxicity.
- 21. (b ) For each toxicology study that is intended primarily to support the safety of the proposed clinical investigation, a full tabulation of data suitable for detailed review. (c) For each nonclinical laboratory study subject to the good laboratory practice regulations under part 58, a statement that the study was conducted in compliance with the good laboratory practice regulations in part 58, or, if the study was not conducted in compliance with those regulations, a brief statement of the reason for the noncompliance.
- 22. IMPORTANCE OF PRECLINICAL TRIALS âĒ To determine the dose, toxic dose, pharmacological action, etc. âĒ It is the requirement of regulatory body for performing clinical trials. âĒ As ethical view point, it is necessary to check safety of drug on animals before starting to check on human being. âĒ To check the kinetic profile of drug & based on it, select the route of administration in human for clinical trials.