Precision and Accuracy General Physics I.pptx
- 5. SPOT THE LIE? IDENTIFY THE LIE FROM THE FOLLOWING STATEMENTS JUDGEMENT IS YOUR BES TWEAPON TO IDENTIFY THE LIE
- 6. ARE YOU READY TO PLAY? LETŌĆÖS START!
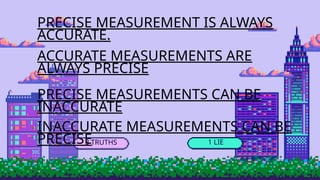
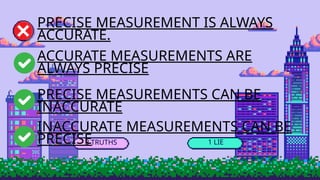
- 7. 3 TRUTHS 1 LIE PRECISE MEASUREMENT IS ALWAYS ACCURATE. ACCURATE MEASUREMENTS ARE ALWAYS PRECISE PRECISE MEASUREMENTS CAN BE INACCURATE INACCURATE MEASUREMENTS CAN BE PRECISE
- 8. 3 TRUTHS 1 LIE PRECISE MEASUREMENT IS ALWAYS ACCURATE. ACCURATE MEASUREMENTS ARE ALWAYS PRECISE PRECISE MEASUREMENTS CAN BE INACCURATE INACCURATE MEASUREMENTS CAN BE PRECISE
- 9. ŌĆó Statement 1: Precision refers to how close multiple measurements of the same quantity are to each other. ŌĆó Statement 2: Accuracy refers to how close a measurement is to the true or accepted value. ŌĆó Statement 3: A measurement can be both precise and accurate if the measurements are close to each other and to the true value. ŌĆó Statement 4: A set of measurements can be precise even if they are all far from the true value, as long as they are close to each other.
- 10. ŌĆó Lie: Statement 4 is a lie. (It's actually true that measurements can be precise but inaccurate if they are close to each other but far from the true value.)
- 11. ŌĆó Statement 1: Systematic errors affect the accuracy of a measurement but do not influence its precision. ŌĆó Statement 2: Random errors affect the precision of a measurement but do not influence its accuracy. ŌĆó Statement 3: Increasing the number of measurements typically improves the precision of the average value. ŌĆó Statement 4: Accuracy can be improved by simply increasing the number of measurements.
- 12. Lie: Statement 4 is a lie. (Increasing the number of measurements improves precision, but not necessarily accuracy, which depends on reducing systematic errors.)
- 13. ŌĆó Statement 1: A measurement device with high precision will always give highly accurate results. ŌĆó Statement 2: Precision is often expressed using standard deviation or variance in a set of measurements. ŌĆó Statement 3: Calibration of instruments is crucial for achieving accurate measurements. ŌĆó Statement 4: Accuracy can be achieved without precision if the measurements are close to the true value but spread out.
- 14. Lie: Statement 1 is a lie. (A precise device does not necessarily give accurate results if it is systematically biased.)
- 15. ŌĆó Statement 1: Precision is more important than accuracy in scientific experiments because reproducibility is key. ŌĆó Statement 2: Accuracy ensures that the experimental results reflect the true values as closely as possible. ŌĆó Statement 3: In the absence of systematic errors, precision and accuracy can both be achieved. ŌĆó Statement 4: A perfectly accurate measurement has zero error.
- 16. Lie: Statement 1 is a lie. (Both precision and accuracy are important in scientific experiments; one cannot be deemed more important than the other universally.)
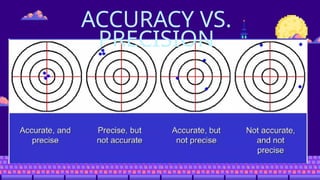
- 18. PRECISION REFERS TO THE UNCERTAINTY IN A MEASUREMENT READING OR OBSERVATION. IT IS CLOSELY LINKED WITH THE TERM ŌĆ£REPRODUCIBILITY.ŌĆØ A PRECISE MEASUREMENT IS ONE WHICH IS CHARACTERIZED BY HIGH REPRODUCIBILITY. REPEATED OBSERVATION LEADS TO NEARLY IDENTICAL REPORTED VALUE
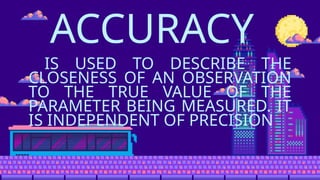
- 19. ACCURACY IS USED TO DESCRIBE THE CLOSENESS OF AN OBSERVATION TO THE TRUE VALUE OF THE PARAMETER BEING MEASURED. IT IS INDEPENDENT OF PRECISION
- 21. UNCERTAINTY
- 22. UNCERTAINTY uncertainty refers to the range within which the true value of a measured quantity is expected to lie. It quantifies the doubt or variation associated with a measurement and indicates the level of confidence in the result.
- 23. ERROR
- 24. ERROR errors refer to the deviations or discrepancies between the measured or observed value and the true or accepted value of a quantity. Errors are inherent in all experimental measurements and can arise from various sources, affecting the accuracy and precision of the results.
- 25. Systematic Error Systematic errors are consistent and repeatable errors that occur due to flaws or biases in the measurement process or instrument.
- 26. Systematic Error Calibration errors, environmental factors (like temperature or humidity affecting measurements), or using an incorrect method or procedure.
- 27. Systematic Error Impact: Systematic errors lead to measurements that are consistently off in the same direction (either too high or too low). They affect the accuracy of the results.
- 28. Systematic Error Detection and Correction: Systematic errors can often be detected by comparing measurements with a known standard or by using different methods to measure the same quantity. Once identified, they can often be corrected or minimized.
- 29. RANDOM ERROR
- 30. RANDOM ERRORS ARE UNPREDICTABLE VARIATIONS THAT OCCUR DURING THE MEASUREMENT PROCESS, CAUSED BY UNPREDICTABLE FACTORS OR INHERENT VARIABILITY IN THE SYSTEM RANDOM ERROR
- 31. FLUCTUATIONS IN THE READINGS OF A BALANCE, SLIGHT DIFFERENCES IN REAGENT AMOUNTS, OR VARIATIONS IN THE ENVIRONMENT THAT ARE NOT CONTROLLED. RANDOM ERROR
- 32. RANDOM ERRORS CAUSE SCATTER IN THE DATA AND AFFECT THE PRECISION OF THE RESULTS. THEY LEAD TO MEASUREMENTS THAT ARE SOMETIMES TOO HIGH AND SOMETIMES TOO LOW, WITH NO CONSISTENT PATTERN. RANDOM ERROR
- 33. REDUCTION: RANDOM ERRORS CAN BE REDUCED BY TAKING MULTIPLE MEASUREMENTS AND AVERAGING THEM, INCREASING THE PRECISION OF THE RESULT. RANDOM ERROR
- 34. MEAN MEAN (ALSO KNOWN AS THE AVERAGE) IS A STATISTICAL MEASURE USED TO DETERMINE THE CENTRAL TENDENCY OF A SET OF DATA POINTS. WHEN IDENTIFYING THE ACCURACY OF DATA MEASURED IN CHEMISTRY, THE MEAN PROVIDES AN ESTIMATE OF THE TRUE
- 35. MEAN The mean is calculated by summing all the individual measurements and then dividing by the number of measurements.
- 36. ACCURACY AND MEAN Accuracy and the Mean: ŌĆó Accuracy refers to how close the mean of the measurements is to the true or accepted value of the quantity being measured. ŌĆó If the mean of the measurements is close to the true value, the data is considered accurate. ŌĆó The mean helps to reduce the effect of random errors, providing a better estimate of the true value compared to a single measurement.
- 37. ACCURACY AND MEAN ŌĆó After calculating the mean, it can be compared to the true or accepted value. The closer the mean is to this true value, the more accurate the measurements are. ŌĆó Systematic Errors: If there is a systematic error in the measurements, the mean may consistently deviate from the true value, indicating a lack of accuracy. ŌĆó Random Errors: While random errors affect individual measurements, they tend to average out when calculating the mean, leading to a more accurate estimate of the true value.
- 38. STANDARD DEVIATION STANDARD DEVIATION IS A STATISTICAL MEASURE THAT QUANTIFIES THE AMOUNT OF VARIATION OR DISPERSION IN A SET OF DATA POINTS. WHEN IDENTIFYING THE PRECISION OF DATA MEASURED IN CHEMISTRY, STANDARD DEVIATION IS A KEY INDICATOR. IT TELLS US HOW MUCH THE INDIVIDUAL MEASUREMENTS DIFFER FROM
- 39. STANDARD DEVIATION AND PRECISION Role of Standard Deviation: ŌĆó The standard deviation provides a numerical value that represents the spread of the measurements around the mean. ŌĆó A small standard deviation indicates that the measurements are close to the mean, meaning high precision. ŌĆó A large standard deviation indicates that the measurements are spread out over a wider range, meaning low precision.
- 40. STANDARD DEVIATION AND PRECISION Interpreting Standard Deviation: ŌĆó Low Standard Deviation: Indicates that the data points are close to the mean, reflecting high precision. This means that the measurements are consistent with each other. ŌĆó High Standard Deviation: Indicates that the data points are spread out from the mean, reflecting low precision. This suggests that the measurements are less consistent.
- 41. THANK YOU AND HAVE A GOOD DAY PLAY AGAIN