Preparing a Standard Solution
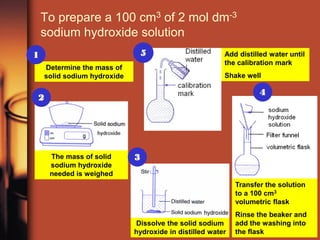
- 1. To prepare a 100 cm3 of 2 mol dm-3 sodium hydroxide solution 1 5 Add distilled water until the calibration mark Determine the mass of solid sodium hydroxide Shake well 4 2 The mass of solid 3 sodium hydroxide needed is weighed Transfer the solution to a 100 cm3 volumetric flask Rinse the beaker and Dissolve the solid sodium add the washing into hydroxide in distilled water the flask
- 2. Prepare a standard solution • To prepare a 100 cm3 of 2 mol dm-3 sodium hydroxide solution • Steps: – Determine the mass of solid NaOH needed – 8.0 g of solid NaOH is weighed in a weighing bottle by using an electronic balance – The solid NaOH is dissolved in about 25 cm3 of water into a beaker – The solution is poured into a 100cm3 volumetric flask.Why step 5 is needed? – The beaker and the weighing bottle are rinsed with distilled water. The washings are then poured into the volumetric flask. – Distilled water is added into the volumetric flask and a dropper is used to add water drop by drop to finally bring the volume of solution to the 100 cm3 mark. – The volumetric flask is stoppered, then inverted and shaken until a homogeneous solution of NaOH is obtained
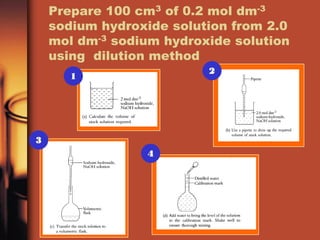
- 3. Prepare a solution with dilution method • To prepare 100 cm3 of 0.20 mol dm-3 sodium hydroxide solution from 2 mol dm-3 sodium hydroxide using dilution method Dilution formula M1V1 = M2V2 M1 = molarity of the solution before dilution V1 = volume of the solution before dilution M2 = molarity of the solution after dilution V2 = volume of the solution after dilution
- 4. Prepare 100 cm3 of 0.2 mol dm-3 sodium hydroxide solution from 2.0 mol dm-3 sodium hydroxide solution using dilution method 2 1 3 4