Presentation on Good Laboratory Practice pptx
- 1. GOOD LABORATORY PRACTICES Presented By: ABDULLA HEL KAFEE Date : 19.09.2021
- 2. The IBN SINA Pharmaceutical Industry Ltd. is committed toŌĆō ’āśServe humanity by manufacturing and providing quality medicines and services to itŌĆÖs customers ; ’āśMaintaining the ethical standard in all itŌĆÖs functions following the requirements of cGMP and regulatory authorities ; ’āś Marching onward for sustainable growth and continual improvement.
- 3. Introduction
- 5. Requirements of GLP ( 21 CFR Part 211, Subpart I- Laboratory Controls )
- 6. Requirements of GLP ( WHO Good practices for pharmaceutical quality control laboratories )
- 7. Requirements of GLP ( ISO/IEC 17025)
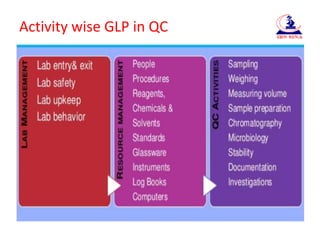
- 8. Activity wise GLP in QC
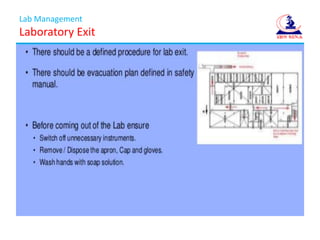
- 10. Lab Management Laboratory Entry ( ContinueŌĆ”)
- 13. Lab Management Laboratory Safety (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 14. Lab Management Laboratory Safety (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 15. Lab Management Laboratory Safety (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 16. Lab Management Laboratory Safety (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 17. Lab Management Laboratory Safety (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 19. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 20. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 21. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
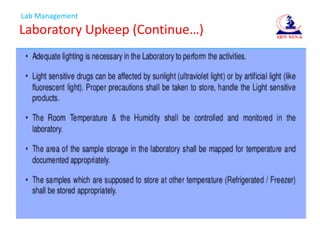
- 22. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 23. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 24. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 25. Lab Management Laboratory Upkeep (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 32. Resource Management Reagents , Chemicals & Solvents
- 33. Resource Management Reagents , Chemicals & Solvents (░õ┤Ū▓į│┘Š▒▓į│▄▒ŌĆ”)
- 41. Resource Management Log Books ( ContinueŌĆ”)
- 69. Thank You Thank you for attending this training course. By keeping the data quality attributes (ALCOA) in the forefront of our minds, we can: Supply safe and effective products to our patients and consumers Maintain compliance so that we can . Keep in mind that the customers and patients relies on us and the accuracy of our data!