Bioenergetics (biochemistry)
- 1. Lee, Thonylet E. Mabinta, Dianne Melad, Maria Fe
- 2. What is Bioenergetics? ď‚›The study of energy in living systems (environments) and the organisms (plants and animals) that utilize them. ď‚›Energy ď‚› Required by all organisms ď‚› May be Kinetic or Potential energy
- 3. Kinetic Energy ď‚›Energy of Motion ď‚›Heat and light energy are examples
- 4. Potential Energy ď‚›Energy of position ď‚›Includes energy stored in chemical bonds
- 5. Bioenergetics ď‚› Bioenergetics is the part of biochemistry concerned with the energy involved in making and breaking of chemical bonds in the molecules found in biological organisms. ď‚› Growth, development and metabolism are some of the central phenomena in the study of biological organisms. The role of energy is fundamental to such biological processes. The ability to harness energy from a variety of metabolic pathways is a property of all living organisms. Life is dependent on energy transformations; living organisms survive because of exchange of energy within and without.
- 6. Bioenergetics ď‚›In a living organism, chemical bonds are broken and made as part of the exchange and transformation of energy. Energy is available for work (such as mechanical work) or for other processes (such as chemical synthesis and anabolic processes in growth), when weak bonds are broken and stronger bonds are made. The production of stronger bonds allows release of usable energy.
- 7. Bioenergetics ď‚›It is the quantitative study of the energy transductions that occur in living cells and of the nature and function of the chemical processes underlying these transductions.
- 8. Law of
- 9. Law of Thermodynamics ď‚›The laws of thermodynamics are important unifying principles of biology. These principles govern the chemical processes (metabolism) in all biological organisms.
- 10. Law of Thermodynamics ď‚› The First Law of Thermodynamics, also know as the law of conservation of energy, states that energy can neither be created nor destroyed. It may change from one form to another, but the energy in a closed system remains constant. ď‚› In any physical or chemical change, the total amount of energy in the universe remains constant, although the form of energy may change.
- 11. Law of Thermodynamics ď‚› The Second Law of Thermodynamics states that when energy is transferred, law of entropy, there will be less energy available at the end of the transfer process than at the beginning. Due to entropy, which is the measure of disorder in a closed system, all of the available energy will not be useful to the organism. Entropy increases as energy is transferred. ď‚› In all natural processes, the entropy of the universe increases.
- 13. ATP Production ď‚›Before cells can use the energy of sunlight or energy /calories stored in carbohydrates, they must transfer the energy to molecules of ATP.
- 14. ATP Production ď‚›ATP is composed of adenine, ribose, and three phosphate groups. ď‚›ATP transfers energy to many different chemical reactions; almost all metabolic pathways directly or indirectly run on energy supplied by ATP.
- 15. ATP Production ď‚›In human cells, cellular respiration releases energy from energy-rich organic molecules and changes ADP into ATP. ď‚›Aerobic respiration is the main ATP- producing pathway ď‚›Anaerobic respiration produces much less ATP (because no oxygen is involved) and can only be used for short periods of time, such as in vigorous muscle exercise.
- 16. ATP Production ď‚›It provides a common energy currency that can be used in many different types of reactions, ď‚›When ATP becomes ADP+P, the amount of energy released is just about enough for the biological purposes, and so little energy is wasted. ď‚›ATP breakdown is coupled to endergonic reactions in such a way that it minimizes energy loss.
- 17. ATP Production ď‚› Initial Breakdown of Glucose ď‚› Glycolysis reactions occur in the cytoplasm (liquid stuff outside the nucleus) and results in the breakdown of glucose to pyruvate; small amounts of ATP are generated. ď‚› Glucose is first phosphorylated in energy-requiring steps then split to form two molecules of PGAL
- 18. ATP Production ď‚› By substrate-level phosphorylation, four ATP are produced; but because two ATP were used previously, there is a net gain of only two ATP. ď‚› Enzymes remove H+ and electrons from PGAL to change NAD to NADH (which is used later in oxidative phosphorylation). ď‚›The end products of glycolysis are: two pyruvates, two ATP (net gain), and two NADH for each glucose molecule degraded.
- 19. ATP Production ď‚›The actual ATP synthesis is accomplished when H ions that have been pumped out of the inner mitochondrial compartment flow back through a channel protein called ATP synthase.
- 20. ATP Production ď‚›Oxygen joins with the "spent" electrons and H+ to yield water. ď‚›The production of ATP is completely dependent on the supply of oxygen that withdraws the electrons at the end of the transport systems.
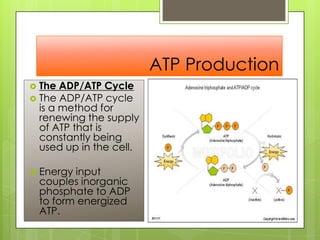
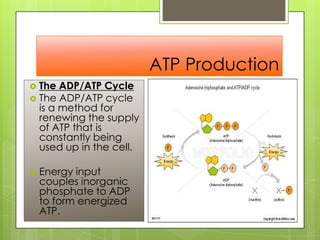
- 21. ATP Production ď‚› The ADP/ATP Cycle ď‚› The ADP/ATP cycle is a method for renewing the supply of ATP that is constantly being used up in the cell. ď‚› Energy input couples inorganic phosphate to ADP to form energized ATP.
- 22. ATP Production ď‚› The ADP/ATP Cycle ď‚› The ADP/ATP cycle is a method for renewing the supply of ATP that is constantly being used up in the cell. ď‚› Energy input couples inorganic phosphate to ADP to form energized ATP.
- 24. ATP Production ď‚› Chemical Work- supplies energy needed to synthesize macromolecules that make up the cell. ď‚› Transport Work- supplies the energy needed to pump substances across the plasma membrane. ď‚› Mechanical Work- supplies the energy needed to permit muscle to contract, cilia and flagella to beat, chromosomes.