Process auditing as per VDA 6.3
- 4. Kiran Walimbe The VDA recommends it’s members to apply VDA 6.3 (2010) standard for the implementation and maintenance of Quality management System, because it believes, higher expectations of products require robust processes which must be secure throughout the entire manufacturing and supply chain. While a System audit of TS 16949 or ISO 9001 seeks out the ‘Conformance’ of the organization to the written standards, the Process audit by VDA 6.3 way verifies the ‘Performance’ of the organization.
- 5. Kiran Walimbe The processes get analyzed in such a way that Risks and Weaknesses are detected in work- processes and their interfaces. The auditor ultimately is attempting to find out whether There is a person responsible for the processes i.e. Process Responsibility PR; Are the Processes directed towards targets based upon the companies requirements i.e. Target orientation TO; is Important information (e.g. quality, problems…) communicated promptly & comprehensively to the necessary persons i.e. Communication CO; and are the Risks in the processes appropriately identified & taken into account i.e. Risk Identification RI.
- 6. Kiran Walimbe PR, TO, CO and RI are the are general requirements relating to a process (Process Orientation) without which the operations & effectiveness of the process in question cannot be guaranteed and the customer interest might be endangered. These elements make up what is known as ‘The Generic Launch Pad’. Amongst other things, the VDA 6.3 process audit ends up assessing the four elements of Generic Launch Pad.
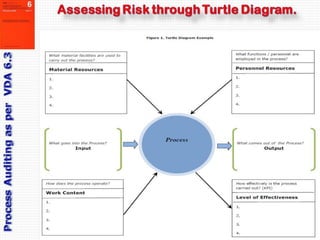
- 8. Kiran Walimbe For auditing the ‘Business Process’ one needs to define a process; identify all the associated risks and in the end evaluate the business process to confirm adequate mitigation of all of these risks. VDA 6.3 defines all business or service processes though plotting of Turtle Diagrams. A typical template is shown here. The risks exist on the interfaces. The auditor first generates a list of possible risks in this interface. Then he makes up a list of Open Questions to seek whether these risks really exists or not.
- 9. Kiran Walimbe He goes to the work place, asks these questions, gets the answers and comes back to convert these open questions to closed questions. He now has got Yes-No answers available to these questions. With this set of derived closed questions he opens his laptop, opens the authorized questionnaire from VDA & records his findings. The macros in the excel sheet do the rest of job. In an answer sheet, he finds the detailed verdict. Not only the averaged cumulative rating is there, but also the systematic derivative in terms of Generic Launch Pad (PR-TO-CO-RI) is available to guide us on what needs to improve in our business set up.
- 10. Kiran Walimbe
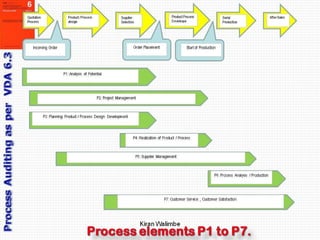
- 11. Kiran Walimbe VDA 6.3 breaks down a typical Product Life Cycle into seven process elements calling them P1 to P7 as shown in diagram. Out of these seven elements, the first one i.e. Potential Analysis P1 applies only while assessing a new and potential supplier. The rest six elements get audited after confirming award of contract & start of regular production.
- 12. Kiran Walimbe The chart here shows the approximate starting and closing points of these six phases in the entire product life-cycle in manufacturing and supply chain; which are: P2-How well is the Project Managed P3-APQP & Planning of Product & Process design & development P4-Product / Process realization or PPAP P5-Effectiveness of Supplier management P6-Process Analysis / Production (Most elaborately done in VDA 6.3) P7-After sales service, resolving customer related issues & customer satisfaction
- 13. Kiran Walimbe Classification Overall level of achievement Eg [%] Description of the classification A Eg > or = 90 Quality capable B 80 = or < Eg < 90 Conditionally quality capable C Eg < 80 Not quality capable At the end of this audit the audited organization may get one of the following ratings.
- 14. Kiran Walimbe Classification Yellow Red Barred RED >14 One question Controlled AMBER Max. 14 None Released GREEN Max. 7 None Special and different treatment to evaluation of new suppliers. The process audit for the process element P1, occurs when a new supplier is identified to meet some need of the organization. The audit Frame work conditions are simple & consist of Confidentiality agreement & Access permission, the Timing is before the award decision and the Risk analysis consists of Identifying product and process risks. Simply put, Potential analysis is nothing but ‘Comparing the performance of similar suppliers with that of the Potential Supplier.’
- 15. Kiran Walimbe P2 for Project Management: There exists a comprehensive project management (including quality planning & risk management) for planning as well as for carrying out the process & product development. The project organization is equipped with the necessary resources, its tasks, authority and expertise are defined and known. The customer is informed of the project planning. A change management (change control) (involving the customer) is established.
- 16. Kiran Walimbe P3 for Planning of project and Product design & development: The requirements necessary for the product & process development are known. The feasibility of the products & the processes to be developed is assured. The product / process development plan ensures that all essential activities are planned with the customer’s agreement. This also includes the need to take into account the necessary technical & personnel resources. Supplier management take into account all designated parts supplied.
- 17. Kiran Walimbe P4 for realization of product / process development: All defined tasks from the planning of the product and process must be conducted in the realization phase. Changes must be recognized & taken into account in the planning. Reviews must be carried out at specified intervals during the realization phase. If target requirements are not achieved, actions must be specified & monitored for effectiveness.
- 18. Kiran Walimbe P5 for Supplier Management: Only approved / released & qualified suppliers are used for serial production processes. Customer requirements are known throughout the supply chain and are implemented. Bought-in products comply with the customer requirements which have been agreed.
- 19. Kiran Walimbe P6 for Process management / Production: This is the element which is audited most intensely. It is further divided into: 6.1 what goes into the process, 6.2 are all production processes controlled? (Process sequence), 6.3 what functions support the process? Personnel resources, 6.4 what facilities are used to achieve the process? (Material resources), 6.5 How effectively is the process carried out? (Effectiveness, efficiency, elimination of waste), 6.6 What should the process produce? (Process result / output)
- 20. Kiran Walimbe P7 for Customer service, Customer satisfaction and Service: The product as delivered meets the customer’s requirements, Customer support and supply of parts are ensured, There are effective and sustainable failure elimination processes in place in the event of complaints and rejects.
- 21. Kiran Walimbe With this set of derived closed questions he opens his laptop, opens the authorized questionnaire from VDA & records his findings. The macros in the excel sheet do the rest of job. In an answer sheet, he finds the detailed verdict. Not only the averaged cumulative rating is there, but also the systematic conclusions in terms of Generic Launch Pad (PR-TO-CO-RI) is available to guide us on what needs to improve in our business set up.
- 22. Kiran Walimbe In case of non-conformances, the ratings of organization can go down. If it was earlier in ‘A’ category, it may get downgraded to either ‘B’ or worse to ‘C’. The remedial course open to the organization consists of following steps: •Get it’s MR team trained in the ways of VDA 6.3 process auditing & commence in-house petrol audits. •Find out where the ratings have suffered losses. Take corrective actions & verify their effectiveness thru’ the teams of senior management. •Decide upon embracing Process Auditing, not to please some external organizations but as means of bringing home true and ever-lasting improvements. After all it is great pride to build a top performing organization & to work in it.
- 23. Kiran Walimbe Those readers, who wish to get formally qualified in auditing by VDA 6.3 (2010) process audit system, can undertake following Training Programs offered by UBF.B Management Pvt. Ltd. Module A + B 2 ....................... Process Auditor for Product Life Cycle (own company and suppliers) Modules B 2............................ Process Auditor for Product Life Cycle (own company and suppliers) Module E (= Modules A + B 2+ C) Certified Process Auditor for Product Life Cycle (any company) Ulrike Peper e-mail Q-training@ubfb.de or e-Mail berlin@ubfb.de I am available for your comments and queries on kiran.walimbe@ubfb.de or kiranwalimbe@live.com











![Kiran Walimbe
Classification Overall level of
achievement Eg
[%]
Description of the
classification
A Eg > or = 90 Quality capable
B 80 = or < Eg < 90 Conditionally
quality capable
C Eg < 80 Not quality
capable
At the end of this audit the audited organization may
get one of the following ratings.](https://image.slidesharecdn.com/cb1c19f7-5d7f-49cf-996b-9d78bd2758e4-150220043155-conversion-gate01/85/Process-auditing-as-per-VDA-6-3-13-320.jpg)






































![vda 6.3 [Autosaved] latest version 2023.pptx](https://cdn.slidesharecdn.com/ss_thumbnails/vda6-240822035548-bed9f315-thumbnail.jpg?width=560&fit=bounds)


















