Protein Synthesis.pptx
- 2. Content • Definition • Location in the cell • Requirements • Steps involved • Initiation • Elongation • Termination • Antibiotics inhibits the protein synthesis
- 3. • The process of protein synthesis translates the codons (nucleotide triplets) of the messenger RNA (mRNA) into the 20-symbol code of amino acids that build the polypeptide chain of the proteins • mRNA translation begins from its 5′-end towards its 3′-end • The polypeptide chain is synthesized from its amino-terminal (N-end) to its carboxyl-terminal (C-end). • no significant differences in the protein synthesis steps in prokaryotes and eukaryotes, however there is one major distinction between the structure of the mRNAs – prokaryotes often have several coding regions (polycistronic mRNA), while the eukaryotic mRNA has only one coding region (monocistronic mRNA
- 4. Protein Synthesis Steps involved • Initiation • Elongation • Termination
- 7. Protein Synthesis Initiation • The components involved in the first step of protein synthesis are: • The mRNA to be translated • the two ribosomal subunits (small and large subunits) • the aminoacyl-tRNA which is specified by the first codon in the mRNA • guanosine triphosphate (GTP), which provides energy for the process – eukaryotes require also adenosine triphosphate! • initiation factors which enables the assembly of this initiation complex - prokaryotes have 3 initiation factors are known (IF-1, IF-2, and IF-3), while eukaryotes, there have over ten factors designated with eIF prefix.
- 9. Two mechanisms are involved in the recognition sequence • Shine-Dalgarno (SD) sequence - In Escherichia coli is observed sequence with high percentage of purine nucleotide bases, known as the Shine-Dalgarno sequence. This region is located close to 5’ end of the mRNA molecule, 6-10 bases upstream of the initiating codon. The 16S rRNA component of the small ribosomal subunit possess a complementary to the SD sequence near its 3'-end.
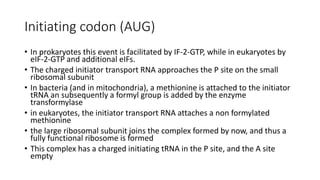
- 10. Initiating codon (AUG) • In prokaryotes this event is facilitated by IF-2-GTP, while in eukaryotes by eIF-2-GTP and additional eIFs. • The charged initiator transport RNA approaches the P site on the small ribosomal subunit • In bacteria (and in mitochondria), a methionine is attached to the initiator tRNA an subsequently a formyl group is added by the enzyme transformylase • in eukaryotes, the initiator transport RNA attaches a non formylated methionine • the large ribosomal subunit joins the complex formed by now, and thus a fully functional ribosome is formed • This complex has a charged initiating tRNA in the P site, and the A site empty
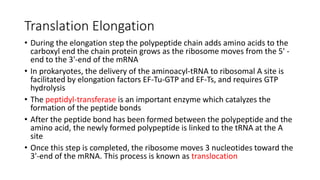
- 11. Translation Elongation • During the elongation step the polypeptide chain adds amino acids to the carboxyl end the chain protein grows as the ribosome moves from the 5' - end to the 3'-end of the mRNA • In prokaryotes, the delivery of the aminoacyl-tRNA to ribosomal A site is facilitated by elongation factors EF-Tu-GTP and EF-Ts, and requires GTP hydrolysis • The peptidyl-transferase is an important enzyme which catalyzes the formation of the peptide bonds • After the peptide bond has been formed between the polypeptide and the amino acid, the newly formed polypeptide is linked to the tRNA at the A site • Once this step is completed, the ribosome moves 3 nucleotides toward the 3'-end of the mRNA. This process is known as translocation
- 13. Termination of Translation • Termination happens when the A site of the ribosome reaches one of the three termination codons (UAA, UAG or UGA) • In prokaryotes, these codons are recognized by different release factors (abbreviated with RF) • When these release factors bind the complex, this cause in hydrolysis of the bond linking the peptide to the tRNA at the P site and releases the nascent protein from the ribosome