QMS importance in Pharma companies
- 1. Presented by :- Vijay Kumar B.Pharmacy (7thsem) 75161827 Presented to :- Mr. Kashish Wilson ( Assistant Professor MMSOP )
- 2. (QMS) ©C Quality management system ©C QMS helps in pharmaceutical to improve the product quality and minimize the risk of product recall ©C Quality management system a set of interacting elements based on procedures , policies ,resources and objectives that are established collectively to guide an organization. Introduction
- 3. Factors essential in developing a process based quality management system in organization (i) Quality Policy (ii) Quality risk management (iii)Quality objectives
- 4. ELEMENTS AND REQUIREMENTS OF A QUALITY MANAGEMENT SYSTEM ©C Quality manual ©C Procedures, instructions, and records ©C Data management ©C Internal processes ©C Customer satisfaction from product quality ©C Improvement opportunities ©C Quality analysis ©C The organizationĪ»s quality policy and quality objectives General elements of QMS includes:
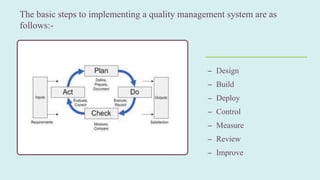
- 5. The basic steps to implementing a quality management system are as follows:- ©C Design ©C Build ©C Deploy ©C Control ©C Measure ©C Review ©C Improve
- 6. List of various data of Quality Management System in filling department of various Pharma Companies? ©C 1) Quality manual. ©C 2) Quality policy ©C 3) Quality procedures ©C 4) Work instructions.
- 7. 1) Quality manual. The manual should fit your organization. The structure and the content of the manual can vary depending on the size of the organization, the complexity of operations, and the competence of the personnel. Small organizations can document the entire QMS in one manual. On the other side, large international organizations may have several different quality manuals. Generally, the manual includes the QMS scope, exclusions from the standard, references to relevant documents, and the business process model.
- 8. 2) Quality policy ©C A policy represents a declarative statement by an organization. A Quality policy should state the commitment of the organization to quality and continual improvement. Usually, this policy is used for promotional purposes and should be displayed in the organizationĪ»s premises and posted on websites, so a clear and short quality policy is convenient and is the general practice. ©C The Quality policy defines the quality objectives to which the organization strives. The quality goals of organizations are defined by quantifying the quality objectives.
- 9. 3) Quality procedures ©C Quality procedures can have different formats and structures. They can be described through text ©C they can be more structured by using tables ©C they can be more illustrative through flow charts ©C they can be any combination of the above.
- 10. Quality procedures should include the following elements: ©C Title , Purpose, Scope ©C Responsibilities and authorities of all people/functions included in any part the procedure ©C Records that result from the activities described in the procedure should be defined and listed; ©C Document control ©C identification of changes, date of review, approval and version of the document should be included in accordance with the established practice for document control. ©C Description of activities ©C this is the main section of the procedure; it relates all the other elements of the procedure and describes what should be done, by whom and how, when and where.
- 11. 4) Work instructions ©C Work instructions can be part of a procedure, or they can be referenced in a procedure. Generally, work instructions have a similar structure to the procedures and cover the same elements; however, the work instructions include details of activities that need to be realized, focusing on the sequencing of the steps, tools, and methods to be used and required accuracy.