Qps Ppt Presentation Sept 2009
- 2. Introduction Quality – Performance – Service Quest Pharmaceutical Services and Bio-Kinetic Clinical Applications merged in early 2008 to form a company based on our mutual beliefs and practices on QUALITY, PERFORMANCE, and SERVICE.
- 3. Location Springfield, Missouri QPS Bio-Kinetic is located just 10 minutes from the Springfield-Branson Regional Airport
- 4. Quality Established Clinical Site • Phase I – IV • Founded in 1994 • Over 800 studies completed State-Of-The-Art Facility • Five (5) Study Units on One Campus • 240 Beds
- 5. Performance State-Of-The-Art Facility • Separate secure pharmacy, retention area, examination rooms, dosing, and phlebotomy stations • State-of-the-art refrigerated centrifuges • High speed micro centrifuges
- 6. Performance State-Of-The-Art Facility • Local clinical lab provides most results within 24 hours a day, 7 days a week • Laminar flow hoods • -70˚C and -20˚C freezers • NIST calibrated temperature recording devices in refrigerators and freezers
- 7. Performance State-Of-The-Art Facility • 24-hour security and monitoring systems • Secure archive/ document storage • FM 200 Gas fire suppression • Automatic natural gas powered generators for power backup • AEDs
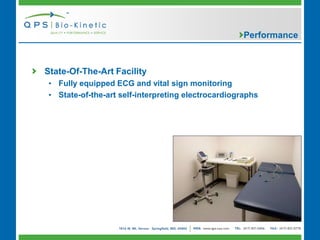
- 8. Performance State-Of-The-Art Facility • Fully equipped ECG and vital sign monitoring • State-of-the-art self-interpreting electrocardiographs
- 9. Quality Clinical Staff Experience • Four (4) Principle Investigators » Two (2) started in 1994 » One (1) started in 2000 » One (1) started 2005 • Clinical Staff Experience » 6+ years average length of employment » Less than 10% staff turnover rate » Leadership team averages over 15 years of experience managing clinical trials
- 10. Quality Audit Experience • Twelve(12) FDA Audits » Ten (10) Clinical Sites » Two (2) IRBs » One 483 (September 2003) » 16 Studies » August 2009
- 11. Service Population Specialties • Healthy normal males • Healthy normal females • Healthy normal birth control free females • Healthy postmenopausal women • Healthy geriatric males and females • Other select populations
- 12. Performance Rapid Recruitment • 500,000 Metropolitan Area Population • 40,000 College Student Population • Over 3 hours from nearest Phase I units • Large Database of High Quality Subjects » Less than 5% drop out rate » Over 20,000 registered subjects in database » Fill 80% of studies exclusively from database
- 13. Performance Rapid Recruitment • Multi-tiered Outreach Effort » Direct – Email & Mail – Phone Calls » Internet – Website – Links from community websites – Interviews
- 14. Performance Rapid Recruitment • Multi-tiered Outreach Effort » Community Events – Job Fair – University Social Events – Promotional Events – Sponsorship » Mass Marketing – Billboards & Buses – TV & Radio – Newspaper Ads » Referral Programs – Participants – Local Business
- 15. Quality Individualized Study Preparation • Protocol Training » Initiation Visit » Protocol Review » Walk Through Meetings – ―Dry Runs‖ – Protocol Training – AE Preparation and Review – Study Events Review » Weekly Staff Review Meeting – Protocol Training – GCP Training – Review Study Preparation – AE Preparation and Review » Custom Communication Plan
- 16. Service Administration Expertise: • Oral • Intravenous • Intramuscular • Intradermal • Subcutaneous • Buccal • Transdermal — cream, gel, patch • Vaginal — cream, gel, suppository • Suppository • Intranasal • Intrauterine
- 17. Performance • Study Integrity » Concise SOPs » Protocol Adaptable Processes » Continual Staff Training » On-Time Study Events » Accurate Sample Collection » Thorough Sample Reconciliation
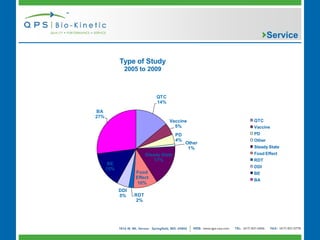
- 18. Service Type of Study 2005 to 2009 QTC 14% BA 27% Vaccine QTC 5% Vaccine PD PD 4% Other Other 1% Steady State Steady State Food Effect 17% RDT BE 15% DDI Food BE Effect BA 10% DDI 5% RDT 2%
- 19. Performance Examples of Study Experience (between 2004 – 2009) • 24 Vaccine Trials » 1 Hepatitis B » 1 Japanese Encephalitis » 1 Yellow Fever » 2 West Nile » 4 Dengue Fever » 5 Small Pox » 10 Flu • 41 Postmenopausal Studies • 34 Geriatric Studies • 19 Birth Control Studies • 12 Cardiac Safety Studies
- 20. Performance Recent Examples of Performance • Study 11708 » 1242 Subjects enrolled in 4 weeks – Aged 40 to 90 – 80% over the age of 60 per protocol » 16,160 Samples processed and shipped on time • Study 11508 » 90 postmenopausal subjects recruited in 4 weeks » Conducted over holiday weekend on time • 7 Studies in same compound » 116 to 140 subjects each » All subjects naïve to compound » Returns out to 3 months » 50/50 male/female » Single IM injection
- 21. Service Collaboration with Quintiles for TQTc • Full service TQTc offering • Ability to conduct large cohorts • High throughput paperless system • 24/7 monitoring by cardiologists • ECG data management from initiation through submission to the FDA ECG Warehouse
- 22. Service QPS • Bioanalysis • Preclinical DMPK • Clinical PK/PD Modeling • Translational Medicine • QPS Taiwan Quality You Expect Service You Trust Turnaround You Need
- 23. Service Commitment to Customer! • Highly experienced staff works to obtain results quickly, on time and on budget • Work with you to meet your objectives • Minimize your costs • Recruit and start your study fast and full • We keep you updated every step of the way Your Success is Our Success!
- 24. Quality "At QPS Bio-Kinetic, you can expect the highest quality and reliable services for your Phase I studies. QPS Bio-Kinetic employs dedicated and experienced people to perform your studies.‖ — Sheela M. Associate Director, QA ―I have found [QPS] Bio-Kinetic to be a highly professional organization with the ability to be flexible and responsive to the needs of the clinical program.‖ — Nicholas P. Associate Director, Early Development ―QPS Bio-Kinetic has the most experience of any Phase I unit in the industry. They have highly-trained, long-term personnel who are not only technically expert but also understand the importance of your study. They have the equation for guaranteed success. I always recommend QPS Bio-Kinetic to my colleagues". — Rick S. CEO
- 25. Quality ―Our company has placed studies at QPS Bio-Kinetic for over 10 years, including the key pharmacokinetic study assessing Drug X components, as well as many of our postmenopausal women, Drug Y pilot bioavailability studies, and all of our pivotal bioequivalence studies. One wouldn't normally think of Springfield, Missouri, as a major CRO site, but the types of subjects they continually enroll are just solid, middle-class citizens with excellent retention for a clinical study. For our pivotal Drug Y BE studies, we routinely enroll over 70 postmenopausal women subjects and no other site in this country or EU can enroll and dose them in one day, as can be done at QPS Bio-Kinetic. QPS Bio-Kinetic is planned as a site for all future Drug Y BE studies. Having served on the Drug Y team and been involved in many management presentations, there is simply no way we can make our timelines and meet our project objectives without using this site.‖ — Phil M. Assistant VP of Clinical Pharmacology
- 26. Quality ―When we look for clinical research sites, we prefer smaller organizations that understand the unique pressures of optimizing speed, quality and price we face as a small company. We demand they be trustworthy, can perform the protocol as directed, are able to communicate study progress and are focused on client service. I have worked with QPS Bio-Kinetic for many years, in particular for BE/BA, DDI and cardiac safety studies. Because of their experienced staff, training and detailed processes, I am confident I can count on qualified study participants, quality data and PK samples to be drawn on time. I trust QPS Bio-Kinetic to tell me when they don’t have the capacity to perform my study according to my specifications or timeline. Based on their history of meeting my expectations and my trust in the QPS Bio-Kinetic staff, QPS Bio-Kinetic has become my first choice as a clinical site.‖ — Jim M. Senior Director, Clinical Development




















![Quality
"At QPS Bio-Kinetic, you can expect the highest quality and reliable services for your
Phase I studies. QPS Bio-Kinetic employs dedicated and experienced people to perform
your studies.‖
— Sheela M.
Associate Director, QA
―I have found [QPS] Bio-Kinetic to be a highly professional organization with the ability
to be flexible and responsive to the needs of the clinical program.‖
— Nicholas P.
Associate Director, Early Development
―QPS Bio-Kinetic has the most experience of any Phase I unit in the industry. They have
highly-trained, long-term personnel who are not only technically expert but also
understand the importance of your study. They have the equation for guaranteed
success. I always recommend QPS Bio-Kinetic to my colleagues".
— Rick S.
CEO](https://image.slidesharecdn.com/qpspptpresentationsept2009-12525974142716-phpapp03/85/Qps-Ppt-Presentation-Sept-2009-24-320.jpg)

