Qualitative analysis of anions
- 1. INORGANIC PRESENTATION TOPIC: THEORITICAL ASPECTS OF ANIONS SUBMITTED BY: NIDHI CHAUDHARY B.Sc(H) CHEMISTRY VI SEMESTER
- 2. QUALITATIVE INORGANIC ANALYSIS It is the analysis (i.e. detection and identification) of the cations and anions present in given compound or salt mixture.
- 3. IDENTIFICATION OF ANIONS AND CATIONS Identification of anions/cations is done through a series of tests, broadly divided into :  Preliminary tests : provide us with the idea of the cation/anion present in the compound.  Confirmatory tests : confirm the presence of the cation/anion in a given compound.
- 4. ANIONS  Anion is an ion with more electrons than protons, giving it a net negative charge (since electrons are negatively charged and protons are positively charged).  Anions are contributed by acids, therefore, they are called as acid radicals. For example, the anion (or acid radical) Cl- in a water solution of salt sodium chloride (NaCl) comes from the hydrochloric acid (HCl) and the cation (or basic radical) Na+ from the base sodium hydroxide (NaOH).
- 5. PREPARING TEST SOLUTIONS Most of the confirmatory tests of the cations/anions are not carried out with the solid mixture but with their solutions :  Water Extract: If the given compound is soluble in water, then 1-2 g of mixture is dissolved in distilled water (10-20 ml).  Sodium Carbonate (soda) Extract: If the compound is insoluble in water, then 1 g of mixture is taken along with 2 g Na2CO3 in distilled water (15-20 ml) and heated for 15 minutes. Solution is filtered and the filterate is soda extract.
- 6. CLASSIFICATION OF ANIONS Anions can not be classified into specific groups but their action with sulphuric acid divides them into three categories:  Dilute Sulphuric Acid: CO 2-, NO -, 3 2 CH3COO-, S2-, SO32-  Conc. Sulphuric Acid: Cl-, Br-, I-, NO -, 3  Independent Radicals: SO 2-,PO 3-,BO 3-, 4 4 3 C2O42-
- 7. SNo EXPERIMENT OBSERVATIONS INFERENCE 1. TEST FOR CARBONATE (CO32-) Pre. Test : To a pinch of Colorless gas evolves May be CO32- . mixture add few drops of with brisk dilute sulphuric acid. effervescence. 2 H+ + CO32- H2O + CO2 Con. Test : Pass the gas Lime water turns evolved in the above step milky. through lime water. M(OH)2 + CO2 MCO3 CO32- + H2O confirmed.
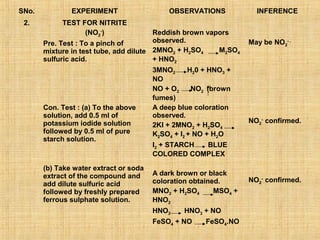
- 8. SNo. EXPERIMENT OBSERVATIONS INFERENCE 2. TEST FOR NITRITE (NO2-) Reddish brown vapors Pre. Test : To a pinch of observed. May be NO2- . mixture in test tube, add dilute 2MNO2 + H2SO4 M2SO4 sulfuric acid. + HNO2 3MNO2 H20 + HNO3 + NO NO + O2 NO2 (brown fumes) Con. Test : (a) To the above A deep blue coloration solution, add 0.5 ml of observed. potassium iodide solution NO2- confirmed. 2KI + 2MNO2 + H2SO4 followed by 0.5 ml of pure K2SO4 + I2 + NO + H2O starch solution. I2 + STARCH BLUE COLORED COMPLEX (b) Take water extract or soda extract of the compound and A dark brown or black coloration obtained. NO2- confirmed. add dilute sulfuric acid followed by freshly prepared MNO2 + H2SO4 MSO4 + ferrous sulphate solution. HNO2 HNO2 HNO3 + NO FeSO4 + NO FeSO4.NO
- 9. SNo. EXPERIMENT OBSERVATIONS INFERENCE 3. TEST FOR ACETATE (CH3COO-) Pre. Test : A pinch of mixture Vinegar like smell is May be CH3COO- . is treated with dilute sulphuric obtained. acid . M(CH3COO) + H2SO4 CH3COOH + MSO4 OR, 1-1.5 g of mixture is taken in Vinegar like smell is watch glass to which a pinch obtained. of oxalic acid and a few drops of water were added. Deep red colored Con. Test: (a) 1 ml of precipitate is observed water/soda extract is due to formation of a neutralized with dilute acetic complex. acid followed by addition of ferric chloride solution. CH3COO- + Fe3+ + H2O CH3COO- confirmed. [Fe3(OH)2(CH3COO)6]+ + 2H+ [Fe3(OH)2(CH3COO)6]+ + H2O CH3COOH + [Fe(OH)2(CH3COO)] + H+
- 10. SNo. EXPERIMENT OBSERVATIONS INFERENCE (b) To 1 ml of water/soda CH3COO- confirmed. Blue color is obtained. extract lithium nitrate solution and potassium iodide solution were added. After this, few drops of dilute ammonia solution was added and the resultant solution was heated. (c) Equal volume of soda/water CH3COO- confirmed. extract and pure calcium Yellow precipitate is hydroxide is taken in a test obtained. tube. Heat the solution and Ca(OH)2 + M(CH3COO) pass the evolved vapors into CH3COCH3 a solution of 2,4 DNP hydrazine. CH3COCH3 + H2NHC6H5(NO2) (CH3)2CNNHC6H5(NH2)2
- 11. SNo. EXPERIMENT OBSERVATIONS INFERENCE 4. TEST FOR SULPHIDE (S2-) Pre. Test: Take a pinch of A colorless gas evolves May be S2- . mixture in a test tube and which smells like rotten add dilute sulphuric acid. egg. S2- + H2SO4 H2S + SO42- Con Test: a) Pass the above Yellow precipitate is gas in cadmium acetate. obtained. Cd(CH3COO)2 + H2S CdS S2- confirmed. + CH3COOH Acidify 1-2 ml of soda/water Black precipitate obtained. (b) extract with dilute acetic H2S + Pb(CH3COO)2 PbS acid and boil the solution to + CH3COOH expel carbon dioxide. To this S2- confirmed. add 1-2 ml of lead acetate solution. Add 1-2 ml of freshly (c) prepared sodium Violet coloration obtained. nitroprusside solution to the S2- + Na2[Fe(CN)5NO] soda/water extract. S2- confirmed. Na4[Fe(CN)5NOS]
- 12. SNO EXPERIMENT OBSERVATIONS INFERENCE 5. TEST FOR SULPHITE (SO32-) Pre. Test: Take a pinch of A colorless and pungent May be SO32- mixture and add 2-3 ml of smelling gas evolves. dilute sulphuric acid. Con.Test: (a) Take 1-2 ml of A green color is obtained. soda/water extract; add 2-3 3Na2SO3 + K2Cr2O7 + 4H2SO4 ml of dilute sulphuric acid Cr2(SO4)3 (Green color) and few drops of potassium SO32- confirmed. dichromate solution. + 3Na2SO4 + 4H2O + K2SO4 (b) Take a pinch of mixture and Sulphur dioxide is evolved. add 2-3 ml of dilute hydrochloric acid. SO32- confirmed. White precipitate is Pass the gas evolved obtained. through lime water.
- 13. SNo EXPERIMENT OBSERVATIONS INFERENCE 6. TEST FOR CHLORIDE (Cl-) Pre Test: To a pinch of Greenish yellow gas having May be Cl- . mixture, add 1-2 ml of conc. pungent smell evolves. sulphuric acid. Dense white fumes observed. Bring a glass rod dipped in NaCl + H2SO4 NaHSO4 + ammonium hydroxide near HCl the mouth of the above test HCl + NH4OH NH4Cl + H2O tube. White precipitate formed Con. Test: (a) To the which is soluble in excess of water/soda extract, add dilute ammonium hydroxide. nitric acid and few drops of AgNO3 + NaCl AgCl + silver nitrate. NaNO3 Cl- confirmed. AgCl + 2NH4OH [Ag(NH3)2Cl] (soluble complex) + 2H2O
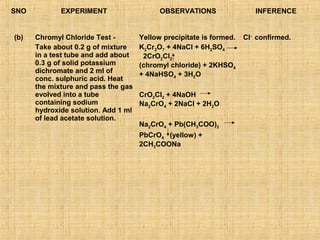
- 14. SNO EXPERIMENT OBSERVATIONS INFERENCE (b) Chromyl Chloride Test - Yellow precipitate is formed. Cl- confirmed. Take about 0.2 g of mixture K2Cr2O7 + 4NaCl + 6H2SO4 in a test tube and add about 2CrO2Cl2 0.3 g of solid potassium (chromyl chloride) + 2KHSO4 dichromate and 2 ml of + 4NaHSO4 + 3H2O conc. sulphuric acid. Heat the mixture and pass the gas evolved into a tube CrO2Cl2 + 4NaOH containing sodium Na2CrO4 + 2NaCl + 2H2O hydroxide solution. Add 1 ml of lead acetate solution. Na2CrO4 + Pb(CH3COO)2 PbCrO4 (yellow) + 2CH3COONa
- 15. SNO EXPERIMENT OBSERVATIONS INFERENCE 7. TEST FOR BROMIDE (Br-) Pre. Test: To a pinch of Reddish brown vapors mixture, add conc. with a pungent smell May be Br-. sulphuric acid. evolved. KBr + H2SO4 KHSO4 + HBr 2HBr + H2SO4 Br2 + SO2 + 2H2O Con Test: (a) Take 0.5 ml of soda/water extract and Yellow precipitate obtained then add 0.5 ml of dilute which is sparingly soluble nitric acid and few drops of in ammonium hydroxide. silver nitrate solution. NaBr + AgNO3 AgBr Br- confirmed. + NaNO3 AgBr + 2NH4OH [Ag(NH3)2]Br + 2H2O
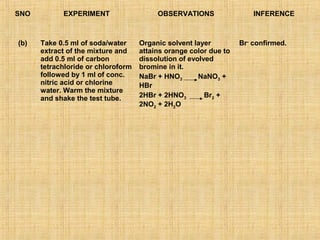
- 16. SNO EXPERIMENT OBSERVATIONS INFERENCE (b) Take 0.5 ml of soda/water Organic solvent layer Br- confirmed. extract of the mixture and attains orange color due to add 0.5 ml of carbon dissolution of evolved tetrachloride or chloroform bromine in it. followed by 1 ml of conc. NaBr + HNO3 NaNO3 + nitric acid or chlorine HBr water. Warm the mixture and shake the test tube. 2HBr + 2HNO3 Br2 + 2NO2 + 2H2O
- 17. SNO EXPERIMENT OBSERVATIONS INFERENCE 8. TEST FOR IODIDE (I-) Pre. Test: To a pinch of Violet vapors of iodine May be I-. mixture, add conc sulphuric evolved due to partial acid. decomposition of the formed HI. KI + H2SO4 KHSO4 + HI 2HI + H2SO4 I2 + SO2 + 2H2O Con Test: (a) Take 0.5 ml of soda/water extract and then Yellow precipitate add 0.5 ml of dilute nitric obtained which is acid followed by 0.5 ml of insoluble in ammonium I- confirmed. silver nitrate solution. hydroxide. NaI + AgNO3 AgI + NaNO3
- 18. SNO EXPERIMENT OBSERVATIONS INFERENCE (b) Take 0.5 ml of soda/water Organic solvent layer I- confirmed. extract of the mixture and attains violet color add 0.5 ml of carbon due to dissolution of tetrachloride or chloroform iodine in it. followed by 1 ml of conc. NaI + HNO3 NaNO3 + Nitric acid or chlorine water. HI Warm the mixture and shake the test tube vigorously. 4HI + O2 2I2 + 2H2O
- 19. SNO EXPERIMENT OBSERVATIONS INFERENCE 9. TEST FOR NITRATE (NO3-) Pre. Test: To a pinch of Light brown fumes May be NO3- mixture, add conc sulphuric observed which intensify acid. on heating the mixture with copper turnings. NaNO3 + H2SO4 NaHSO4 + HNO3 4HNO3 4NO2 + O2 + 2H2O Con Test: Take around 1 ml A brown black ring at the of soda/water extract and junction of two layers is acidify with dilute sulphuric formed. acid. Add 2-3 ml of freshly NaNO3 + H2SO4 NO3- confirmed. prepared ferrous sulphate NaHSO4 + HNO3 solution and then pour 2HNO3 + 6FeSO4 + 3H2SO4 conc. sulphuric acid slowly in thin stream along the 3Fe2(SO4)3 + 2NO + sides of the test tube. 4H2O FeSO4 + NO FeSO4.NO(brown ring)
- 20. SNO EXPERIMENT OBSERVATIONS INFERENCE 10. TEST FOR OXALATE (C2O42-) Pre. Test: Add conc Evolved gas burns with a May be C2O42-. sulphuric acid(1-2 ml) to a blue flame. pinch of given mixture(0.1 g) (COONa)2 + H2SO4 and heat the solution. . Na2SO4 + (COOH)2 Bring the mouth of the test (COOH)2 CO + CO2 + H2O tube near the flame. Also pass the gas in the lime water. Lime water turns milky. Con Test: (a) Acidify 1 ml of soda/water extract of the White precipitate obtained. mixture with dilute acetic (COONa)2 + H2SO4 acid and boil the solution. Na2SO4 + (COOH)2 Then add 1 ml of calcium chloride solution and shake (COOH)2 + CaCl2 the test tube. (COO)2Ca + 2HCl
- 21. SNO EXPERIMENT OBSERVATIONS INFERENCE Dissolve the precipitate in Pink color of potassium C2O42- confirmed. permanganate gets minimum quantity of dilute discharged. sulphuric acid and then add potassium permanganate (COO)2Ca + H2SO4 solution drop wise. (COOH)2 + CaSO4 2KMnO4 + 3H2SO4 + 5(COOH)2 2MnSO4 + K2SO4 + 10CO2 + 8H2O (b) Acidify soda/water extract with dilute acetic acid and Yellow precipitate boil the solution. Add ferrous obtained. sulphate solution. C2O42- confirmed. C2O42- + Fe2+ [Fe(C2O4)]2-
- 22. SNO EXPERIMENT OBSERVATIONS INFERENCE 11. TEST FOR FLUORIDE (F-) Pre. Test: Take 0.1 g of the Oily droplets are formed May be F- mixture and add 1 ml of along the sides of the test tube. conc. sulphuric acid. 2NaF + H2SO4 Na2SO4 + 2HF Con Test: Add a pinch of White gelatinous deposit sand to the above reaction on the glass rod observed. mixture. Heat it. Bring a F- confirmed. 4NaF + H2SO4 + SiO2 glass rod moistened with water near the mouth of SiF4 + 2Na2SO4 + 2H2O the test tube. 3SiF4 + 4H2O H4SiO4 + 2H2SiF6 (hydrofluorosilicic acid; waxy appearance)
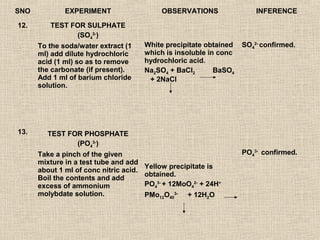
- 23. SNO EXPERIMENT OBSERVATIONS INFERENCE 12. TEST FOR SULPHATE (SO42-) To the soda/water extract (1 White precipitate obtained SO42- confirmed. ml) add dilute hydrochloric which is insoluble in conc acid (1 ml) so as to remove hydrochloric acid. the carbonate (if present). Na2SO4 + BaCl2 BaSO4 Add 1 ml of barium chloride + 2NaCl solution. 13. TEST FOR PHOSPHATE (PO43-) Take a pinch of the given PO43- confirmed. mixture in a test tube and add Yellow precipitate is about 1 ml of conc nitric acid. obtained. Boil the contents and add excess of ammonium PO43- + 12MoO42- + 24H+ molybdate solution. PMo12O403- + 12H2O
- 24. SNO EXPERIMENT OBSERVATIONS INFERENCE 14. TEST FOR BORATE (BO33-) In a porcelain dish, take a Green flame is observed. BO33- confirmed. small amount of the mixture, Na3BO3 + 3H2SO4 say 0.2-0.3 g, and add nearly Na2SO4 + 2H3BO3 1 ml of conc sulphuric acid followed by 1 ml of ethyl alcohol. Heat the dish and H3BO3 + C2H5OH bring a burning paper over B(OC2H5)3 + H2O it.







![SNo. EXPERIMENT OBSERVATIONS INFERENCE
3. TEST FOR ACETATE
(CH3COO-)
Pre. Test : A pinch of mixture Vinegar like smell is May be CH3COO- .
is treated with dilute sulphuric obtained.
acid . M(CH3COO) + H2SO4
CH3COOH + MSO4
OR,
1-1.5 g of mixture is taken in Vinegar like smell is
watch glass to which a pinch obtained.
of oxalic acid and a few drops
of water were added.
Deep red colored
Con. Test: (a) 1 ml of precipitate is observed
water/soda extract is due to formation of a
neutralized with dilute acetic complex.
acid followed by addition of
ferric chloride solution. CH3COO- + Fe3+ + H2O CH3COO- confirmed.
[Fe3(OH)2(CH3COO)6]+ +
2H+
[Fe3(OH)2(CH3COO)6]+ +
H2O CH3COOH +
[Fe(OH)2(CH3COO)] + H+](https://image.slidesharecdn.com/qualitativeanalysis-130215085936-phpapp01/85/Qualitative-analysis-of-anions-9-320.jpg)

![SNo. EXPERIMENT OBSERVATIONS INFERENCE
4. TEST FOR SULPHIDE
(S2-)
Pre. Test: Take a pinch of A colorless gas evolves May be S2- .
mixture in a test tube and which smells like rotten
add dilute sulphuric acid. egg.
S2- + H2SO4 H2S + SO42-
Con Test: a) Pass the above Yellow precipitate is
gas in cadmium acetate. obtained.
Cd(CH3COO)2 + H2S CdS
S2- confirmed.
+ CH3COOH
Acidify 1-2 ml of soda/water Black precipitate obtained.
(b) extract with dilute acetic H2S + Pb(CH3COO)2 PbS
acid and boil the solution to + CH3COOH
expel carbon dioxide. To this S2- confirmed.
add 1-2 ml of lead acetate
solution.
Add 1-2 ml of freshly
(c) prepared sodium Violet coloration obtained.
nitroprusside solution to the S2- + Na2[Fe(CN)5NO]
soda/water extract. S2- confirmed.
Na4[Fe(CN)5NOS]](https://image.slidesharecdn.com/qualitativeanalysis-130215085936-phpapp01/85/Qualitative-analysis-of-anions-11-320.jpg)

![SNo EXPERIMENT OBSERVATIONS INFERENCE
6. TEST FOR CHLORIDE
(Cl-)
Pre Test: To a pinch of Greenish yellow gas having May be Cl- .
mixture, add 1-2 ml of conc. pungent smell evolves.
sulphuric acid. Dense white fumes observed.
Bring a glass rod dipped in NaCl + H2SO4 NaHSO4 +
ammonium hydroxide near HCl
the mouth of the above test
HCl + NH4OH NH4Cl + H2O
tube.
White precipitate formed
Con. Test: (a) To the which is soluble in excess of
water/soda extract, add dilute ammonium hydroxide.
nitric acid and few drops of AgNO3 + NaCl AgCl +
silver nitrate. NaNO3
Cl- confirmed.
AgCl + 2NH4OH
[Ag(NH3)2Cl] (soluble
complex) + 2H2O](https://image.slidesharecdn.com/qualitativeanalysis-130215085936-phpapp01/85/Qualitative-analysis-of-anions-13-320.jpg)
![SNO EXPERIMENT OBSERVATIONS INFERENCE
7. TEST FOR BROMIDE
(Br-)
Pre. Test: To a pinch of Reddish brown vapors
mixture, add conc. with a pungent smell May be Br-.
sulphuric acid. evolved.
KBr + H2SO4 KHSO4 +
HBr
2HBr + H2SO4 Br2 + SO2
+ 2H2O
Con Test: (a) Take 0.5 ml of
soda/water extract and Yellow precipitate obtained
then add 0.5 ml of dilute which is sparingly soluble
nitric acid and few drops of in ammonium hydroxide.
silver nitrate solution. NaBr + AgNO3 AgBr Br- confirmed.
+ NaNO3
AgBr + 2NH4OH
[Ag(NH3)2]Br + 2H2O](https://image.slidesharecdn.com/qualitativeanalysis-130215085936-phpapp01/85/Qualitative-analysis-of-anions-15-320.jpg)




![SNO EXPERIMENT OBSERVATIONS INFERENCE
Dissolve the precipitate in Pink color of potassium C2O42- confirmed.
permanganate gets
minimum quantity of dilute
discharged.
sulphuric acid and then add
potassium permanganate (COO)2Ca + H2SO4
solution drop wise. (COOH)2 + CaSO4
2KMnO4 + 3H2SO4 +
5(COOH)2 2MnSO4 +
K2SO4 + 10CO2 + 8H2O
(b) Acidify soda/water extract
with dilute acetic acid and Yellow precipitate
boil the solution. Add ferrous obtained.
sulphate solution. C2O42- confirmed.
C2O42- + Fe2+
[Fe(C2O4)]2-](https://image.slidesharecdn.com/qualitativeanalysis-130215085936-phpapp01/85/Qualitative-analysis-of-anions-21-320.jpg)