QUANTUM MECHANICAL MODEL OF THE ATOM.pptx
Download as PPTX, PDF0 likes15 views
The document discusses the quantum mechanical model of the atom, which describes atoms as having a positively charged nucleus surrounded by negatively charged electrons, with insights from physicists de Broglie, Schr├Čdinger, and Heisenberg. It explains that electrons occupy regions called atomic orbitals, where their positions can only be determined probabilistically, and outlines the structure of principal energy levels and sublevels. The document emphasizes the mathematical foundation of the model and its implications for understanding electron configuration and energy levels.
1 of 23
Download to read offline














Ad
Recommended
06_ Electronic Structure of an Atom.pptx
06_ Electronic Structure of an Atom.pptxM Ag
╠²
The document provides a thorough overview of quantum mechanics and atomic structure, detailing the composition of atoms, quantum numbers, and electron configurations. It discusses Schr├ČdingerŌĆÖs quantum mechanical model, which depicts electrons as being part of a probability cloud rather than defined orbits. Key concepts such as energy levels, orbitals, and quantum numbers are explained, highlighting the principles governing atomic and subatomic particles.GenChem - Electronic Structure of Atoms
GenChem - Electronic Structure of AtomsReid Manares
╠²
The document provides an overview of the quantum mechanical description of the atom, including Schr├Čdinger's model of the hydrogen atom, quantum numbers, and electron configuration. It explains the structure of atoms, detailing the composition of protons, neutrons, and electrons, while introducing core principles of quantum mechanics, such as the Heisenberg uncertainty principle and the Schr├Čdinger equation. Key concepts like energy levels, sublevels, orbitals, and quantum numbers that describe electron behavior are also discussed.1-Quantum-Mechanical-Model-and-Energy-Levels.pptx
1-Quantum-Mechanical-Model-and-Energy-Levels.pptxJessieForsuelo
╠²
The document discusses the quantum mechanical model of the atom, which explains electron positions and energy levels using probability clouds rather than fixed orbits. Key points include the principal quantum number (n), the existence of different sublevels, and the distribution of electrons among energy levels, emphasizing that higher levels can hold more electrons and that energy increases with distance from the nucleus. It contrasts this model with earlier atomic theories, notably the Bohr model, highlighting its accuracy and acceptance in modern science.Chap 5 ppt chemistry 2016
Chap 5 ppt chemistry 2016reginagreene
╠²
The document discusses the development of atomic models from Dalton to the modern Quantum Mechanical Model. It describes J.J. Thomson's "plum pudding" model, Rutherford's discovery of the nucleus, and Bohr's model of electron orbits. The key developments were the proposals that electrons exist in specific energy levels and orbitals, and that their behavior and location can only be described statistically through quantum mechanics rather than with definite paths. The Quantum Mechanical Model provides a mathematical description of electron energies and probabilities using the Schrodinger equation.G9 Science Q2- Week 1- Quantum (1).pptx
G9 Science Q2- Week 1- Quantum (1).pptxroselindolos
╠²
The document discusses the structure of an atom, including its subatomic particles (protons, neutrons, electrons) and models (Bohr and quantum mechanical models). It covers the historical development of atomic theory, highlighting contributions from scientists like Dalton, Thomson, and Rutherford, while explaining concepts such as energy levels, electronic configuration, and the behavior of electrons. The document also emphasizes the limitations of earlier models and the emergence of the quantum mechanical model for explaining atomic characteristics and the emission of light.Quantum-Mechanical-Model-of-the-Atom-shortcut.pdf
Quantum-Mechanical-Model-of-the-Atom-shortcut.pdfdenveralbarico143
╠²
The document discusses the evolution of atomic models, focusing on the quantum mechanical model developed by notable physicists such as Bohr and de Broglie. It outlines the limitations of previous atomic models, particularly BohrŌĆÖs model, which only accurately describes hydrogen's emission spectrum and does not account for the wave-like behavior of electrons. The quantum mechanical model introduces orbitals, representing probabilities of electron locations and accommodating energy principles involving quantized levels and uncertainty in measurement.Science | Grade 9 - 2nd Quarter
Science | Grade 9 - 2nd QuarterThe Fusion
╠²
The document details the history and development of atomic models from ancient concepts by Democritus to modern quantum mechanical theories, highlighting key figures like Dalton, Thomson, Rutherford, Bohr, Sommerfeld, Schr├Čdinger, and Chadwick. It discusses the structure of atoms, including subatomic particles (protons, neutrons, and electrons), their properties, and the principles guiding electron behavior and chemical bonding. Additional mentions include various theories related to electron configuration, atomic mass, and types of chemical bonds.Models
ModelsGalen West
╠²
The document discusses the evolution of atomic models over time from Dalton's model to the current quantum mechanical model. It summarizes key developments including the plum pudding model, Bohr's model of electrons in orbits, and how the Schrodinger equation led to the quantum mechanical model where electrons occupy distinct energy levels and orbitals. The modern model describes electron probability clouds rather than set orbits and accounts for properties like electron spin and the Pauli exclusion principle.Quantum Theory and the Atom.ppt science 9x
Quantum Theory and the Atom.ppt science 9xAprilJoyMangurali1
╠²
The document discusses the quantum mechanical model of the atom and how it describes the positions and energies of electrons in atoms. It explains that Bohr's early model of discrete electron orbits was replaced by the quantum model, where electrons exist as probabilistic wave functions rather than definite orbits. The quantum model defines principal and subenergy levels associated with different atomic orbitals that electrons can occupy, specified by quantum numbers like n, l, and m. This explains the observed structure of elements and periodic table.Electrons in Atoms
Electrons in AtomsCurrituck County High School
╠²
The document discusses the development of atomic models from Dalton to Bohr and beyond. It describes Rutherford's discovery of the nucleus and Bohr's model of electrons in fixed orbits around the nucleus. Later, the quantum mechanical model was developed, restricting electrons to specific energy levels rather than exact orbits. This modern model determines the probability of finding electrons in different locations around the nucleus.SCIENCE 9-LESSON 10yehheeeeyyeyeyeyye.pdf
SCIENCE 9-LESSON 10yehheeeeyyeyeyeyye.pdfaflores17
╠²
The quantum mechanical model of the atom updates the Bohr model by describing electrons as existing in three-dimensional regions called atomic orbitals rather than circular orbits. Atomic orbitals describe the probable location of electrons and differ in energy and shape. The model was developed based on the work of de Broglie, Heisenberg, and Schr├Čdinger and their discoveries about the wave-particle duality of electrons and Heisenberg's uncertainty principle. It describes the organization of electrons in atoms using principal energy levels, sublevels that define orbital shape, individual orbitals within sublevels, and electron spin.Lesson 1 - GRADE 9 Carbon (Science).pptx
Lesson 1 - GRADE 9 Carbon (Science).pptxDoveLalune
╠²
The document outlines the development of the quantum mechanical model of the atom, focusing on how it describes the energies and positions of electrons through various quantum numbers. Key contributions from physicists like Niels Bohr, Erwin Schr├Čdinger, and Werner Heisenberg are highlighted, including the uncertainty principle and the mathematical equations that define electron behavior. Additionally, the document includes a group activity and assessments related to quantum numbers and their connection to the periodic table.Electrons in atoms notes
Electrons in atoms notesmartykilroy
╠²
The document summarizes the development of atomic models from Rutherford to the current quantum mechanical model. It discusses inadequacies in Rutherford's model that could not explain atomic properties and emissions. Bohr proposed electrons orbit in distinct energy levels, but this failed to explain multi-electron atoms. The quantum mechanical model treats electrons as waves using Schrodinger's equation, describing electrons as probability distributions rather than particles. It introduces quantum numbers to characterize electron states and explains how orbitals are filled according to various rules.atomic_structure.pdf
atomic_structure.pdfReneeRamdial3
╠²
An electron moving with a speed of some value has a de Broglie wavelength that can be calculated using de Broglie's wave equation. De Broglie proposed that particles like electrons can behave as waves, with a wavelength inversely proportional to its momentum. The document provides background on atomic structure and quantum theory concepts like quantized energy levels and wave-particle duality.Electronic-Structure-of-Atom-Part-I (2).pptx
Electronic-Structure-of-Atom-Part-I (2).pptxJhosenRamos
╠²
The document describes the quantum mechanical model of the atom. It states that the model views electrons as existing in clouds or orbitals around the nucleus, with higher probability of finding electrons in regions called principal energy levels or shells. Electrons in inner shells are held more tightly and have lower energy than those in outer shells. Shells are divided into subshells which are further divided into orbitals that can each hold two electrons. Electrons fill the lowest energy orbitals first according to the Aufbau principle.Powerpoint_5.1.ppt
Powerpoint_5.1.pptFrenzDelaCruz2
╠²
The document discusses the development of atomic models from Rutherford's model to Bohr's model to the modern quantum mechanical model. It explains that Bohr's model improved upon Rutherford's by incorporating the idea that electrons orbit in distinct energy levels. However, the quantum mechanical model determines allowed electron energies and locations using probability distributions rather than distinct orbits. It also describes the structures of atomic orbitals that make up different energy levels.Powerpoint_5.1.ppt
Powerpoint_5.1.pptLily187094
╠²
The document discusses the development of atomic models from Rutherford's model to Bohr's model to the modern quantum mechanical model. It explains that Bohr's model improved upon Rutherford's by incorporating the idea that electrons orbit in distinct energy levels. However, the quantum mechanical model determines allowed electron energies and locations as probabilities rather than precise orbits. It also describes atomic orbitals as regions of high probability for electron location.Atomic structure & chemical bond
Atomic structure & chemical bondSabbir Ahmed
╠²
1) The presentation summarizes atomic structure and chemical bonding models including fundamental particles, Rutherford and Bohr atomic models, quantum numbers, and types of chemical bonds.
2) It discusses limitations of earlier atomic models and introduces concepts like electron energy levels, photon energy, and spectral lines.
3) Various types of chemical bonds are defined including ionic, covalent, and metallic bonds. Molecular orbital theory of covalent bonding is also introduced.Qrt-2-Week-1-Module-1-Lesson-1.pptx
Qrt-2-Week-1-Module-1-Lesson-1.pptxMATRIXGAMING8
╠²
This document provides an overview of the quantum mechanical model of the atom. It discusses key concepts like the development of atomic models, electron configuration, quantum numbers, electron orbitals, and energy levels. Some key points:
- The quantum mechanical model describes the probable locations of electrons in an atom and how they can have discrete energy levels. It improved upon earlier planetary and Bohr models.
- Electrons occupy specific orbitals and energy levels denoted by quantum numbers like principal and azimuthal quantum numbers.
- Electron configuration notation specifies the distribution of electrons across orbitals based on Aufbau's principle and other rules.
- Transitions between energy levels explain the emission or absorption of photons and thus the colorsSolid state physics
Solid state physicsimtiazalijoono
╠²
The document discusses the fundamental concepts of solid-state physics, focusing on the atomic structure and the differences between conductors, semiconductors, and insulators. It covers the Bohr model, quantum model, and the significance of valence and free electrons in electrical conduction. Additionally, it explains the band gap and the properties of materials used in electronics.TOP ICSE SCHOOLS IN DELHI NCR
TOP ICSE SCHOOLS IN DELHI NCRScholars Learning
╠²
1) The document discusses the structure and constituents of atoms, including the discovery of electrons, protons, and neutrons through experiments.
2) It describes models of the atom including Thomson's "plum pudding" model, Rutherford's nuclear model, Bohr's model incorporating stationary electron orbits, and the quantum mechanical model involving orbitals and energy levels.
3) Key concepts discussed include the dual wave-particle nature of matter and radiation, Planck's quantum theory and photon concept, and the photoelectric effect.Chapter 1_Atomic Structure_PDF_GENERAL CHEMISTRY
Chapter 1_Atomic Structure_PDF_GENERAL CHEMISTRYMinhQuan85
╠²
The document provides an overview of atomic structure, detailing historical developments in atomic theory from Dalton to Schr├Čdinger and discussing key concepts such as atomic models, subatomic particles, quantum numbers, and the Schr├Čdinger wave equation. It outlines significant findings related to atomic particles including electrons, protons, and neutrons, and explains various models including Bohr's model and the modern quantum mechanical model. Additionally, it touches on wave-particle duality and the significance of quantum numbers in determining the state of electrons in an atom.Quantum mechanical model
Quantum mechanical modelIlac Bernardo
╠²
The document outlines the quantum mechanical model of the atom, detailing the concept of quanta and the contributions of physicists like Max Planck, Albert Einstein, and Niels Bohr to our understanding of atomic structure. It explains how electrons occupy quantized energy levels and introduces quantum numbers to describe their positions and behaviors within an atom. Additionally, it highlights significant contributions from key figures in quantum mechanics, including the development of foundational theories such as wave mechanics and the uncertainty principle.Atomic Models.pptx
Atomic Models.pptxArnelButlig
╠²
Democritus first proposed in 400 BC that matter is made of indivisible particles called atoms. This idea was ignored for over 2000 years. In the early 1800s, Dalton proposed that atoms are small, hard spheres that make up elements. Thomson's 1897 discovery of the electron led to his "plum pudding" model of atoms with positive charge and embedded electrons. Rutherford's 1909 gold foil experiment showed that atoms have a small, dense nucleus surrounded by empty space. Bohr's 1913 model depicted electrons orbiting the nucleus in fixed energy levels like planets around the sun. Modern atomic theory describes electrons as existing in probabilistic "clouds" or orbitals around the nucleus based on quantum mechanics.TOP ICSE SCHOOLS IN DELHI NCR
TOP ICSE SCHOOLS IN DELHI NCRScholars Learning
╠²
The document outlines the structure of the atom, detailing the discoveries of electrons, protons, and neutrons, as well as various atomic models including Thomson's, Rutherford's, and Bohr's models. It discusses concepts such as atomic number, mass number, electromagnetic wave theory, and introduces Planck's quantum theory to explain phenomena like the photoelectric effect. The document also covers advanced topics like the Heisenberg uncertainty principle, de Broglie's dual nature of matter, and the quantum mechanical model of the atom, including orbital shapes and energy levels.Quantum theory and the╠²atom
Quantum theory and the╠²atomKamal Metwalli
╠²
This document provides an overview of Bohr's model of the atom and the development of the quantum mechanical model. It discusses key aspects of Bohr's model including discrete energy levels and allowed electron orbits. It then describes limitations of Bohr's model and the proposal of the quantum mechanical model treating electrons as waves. The quantum mechanical model uses wave functions and orbitals to describe electron location probabilities rather than defined orbits. It introduces the concepts of principal and subenergy levels characterized by quantum numbers that specify allowed electron configurations.Electron configuration-Grade 9 Science.pptx
Electron configuration-Grade 9 Science.pptxAlvinJuanino
╠²
Electron configuration-Grade 9 Science.pptxMore Related Content
Similar to QUANTUM MECHANICAL MODEL OF THE ATOM.pptx (20)
Models
ModelsGalen West
╠²
The document discusses the evolution of atomic models over time from Dalton's model to the current quantum mechanical model. It summarizes key developments including the plum pudding model, Bohr's model of electrons in orbits, and how the Schrodinger equation led to the quantum mechanical model where electrons occupy distinct energy levels and orbitals. The modern model describes electron probability clouds rather than set orbits and accounts for properties like electron spin and the Pauli exclusion principle.Quantum Theory and the Atom.ppt science 9x
Quantum Theory and the Atom.ppt science 9xAprilJoyMangurali1
╠²
The document discusses the quantum mechanical model of the atom and how it describes the positions and energies of electrons in atoms. It explains that Bohr's early model of discrete electron orbits was replaced by the quantum model, where electrons exist as probabilistic wave functions rather than definite orbits. The quantum model defines principal and subenergy levels associated with different atomic orbitals that electrons can occupy, specified by quantum numbers like n, l, and m. This explains the observed structure of elements and periodic table.Electrons in Atoms
Electrons in AtomsCurrituck County High School
╠²
The document discusses the development of atomic models from Dalton to Bohr and beyond. It describes Rutherford's discovery of the nucleus and Bohr's model of electrons in fixed orbits around the nucleus. Later, the quantum mechanical model was developed, restricting electrons to specific energy levels rather than exact orbits. This modern model determines the probability of finding electrons in different locations around the nucleus.SCIENCE 9-LESSON 10yehheeeeyyeyeyeyye.pdf
SCIENCE 9-LESSON 10yehheeeeyyeyeyeyye.pdfaflores17
╠²
The quantum mechanical model of the atom updates the Bohr model by describing electrons as existing in three-dimensional regions called atomic orbitals rather than circular orbits. Atomic orbitals describe the probable location of electrons and differ in energy and shape. The model was developed based on the work of de Broglie, Heisenberg, and Schr├Čdinger and their discoveries about the wave-particle duality of electrons and Heisenberg's uncertainty principle. It describes the organization of electrons in atoms using principal energy levels, sublevels that define orbital shape, individual orbitals within sublevels, and electron spin.Lesson 1 - GRADE 9 Carbon (Science).pptx
Lesson 1 - GRADE 9 Carbon (Science).pptxDoveLalune
╠²
The document outlines the development of the quantum mechanical model of the atom, focusing on how it describes the energies and positions of electrons through various quantum numbers. Key contributions from physicists like Niels Bohr, Erwin Schr├Čdinger, and Werner Heisenberg are highlighted, including the uncertainty principle and the mathematical equations that define electron behavior. Additionally, the document includes a group activity and assessments related to quantum numbers and their connection to the periodic table.Electrons in atoms notes
Electrons in atoms notesmartykilroy
╠²
The document summarizes the development of atomic models from Rutherford to the current quantum mechanical model. It discusses inadequacies in Rutherford's model that could not explain atomic properties and emissions. Bohr proposed electrons orbit in distinct energy levels, but this failed to explain multi-electron atoms. The quantum mechanical model treats electrons as waves using Schrodinger's equation, describing electrons as probability distributions rather than particles. It introduces quantum numbers to characterize electron states and explains how orbitals are filled according to various rules.atomic_structure.pdf
atomic_structure.pdfReneeRamdial3
╠²
An electron moving with a speed of some value has a de Broglie wavelength that can be calculated using de Broglie's wave equation. De Broglie proposed that particles like electrons can behave as waves, with a wavelength inversely proportional to its momentum. The document provides background on atomic structure and quantum theory concepts like quantized energy levels and wave-particle duality.Electronic-Structure-of-Atom-Part-I (2).pptx
Electronic-Structure-of-Atom-Part-I (2).pptxJhosenRamos
╠²
The document describes the quantum mechanical model of the atom. It states that the model views electrons as existing in clouds or orbitals around the nucleus, with higher probability of finding electrons in regions called principal energy levels or shells. Electrons in inner shells are held more tightly and have lower energy than those in outer shells. Shells are divided into subshells which are further divided into orbitals that can each hold two electrons. Electrons fill the lowest energy orbitals first according to the Aufbau principle.Powerpoint_5.1.ppt
Powerpoint_5.1.pptFrenzDelaCruz2
╠²
The document discusses the development of atomic models from Rutherford's model to Bohr's model to the modern quantum mechanical model. It explains that Bohr's model improved upon Rutherford's by incorporating the idea that electrons orbit in distinct energy levels. However, the quantum mechanical model determines allowed electron energies and locations using probability distributions rather than distinct orbits. It also describes the structures of atomic orbitals that make up different energy levels.Powerpoint_5.1.ppt
Powerpoint_5.1.pptLily187094
╠²
The document discusses the development of atomic models from Rutherford's model to Bohr's model to the modern quantum mechanical model. It explains that Bohr's model improved upon Rutherford's by incorporating the idea that electrons orbit in distinct energy levels. However, the quantum mechanical model determines allowed electron energies and locations as probabilities rather than precise orbits. It also describes atomic orbitals as regions of high probability for electron location.Atomic structure & chemical bond
Atomic structure & chemical bondSabbir Ahmed
╠²
1) The presentation summarizes atomic structure and chemical bonding models including fundamental particles, Rutherford and Bohr atomic models, quantum numbers, and types of chemical bonds.
2) It discusses limitations of earlier atomic models and introduces concepts like electron energy levels, photon energy, and spectral lines.
3) Various types of chemical bonds are defined including ionic, covalent, and metallic bonds. Molecular orbital theory of covalent bonding is also introduced.Qrt-2-Week-1-Module-1-Lesson-1.pptx
Qrt-2-Week-1-Module-1-Lesson-1.pptxMATRIXGAMING8
╠²
This document provides an overview of the quantum mechanical model of the atom. It discusses key concepts like the development of atomic models, electron configuration, quantum numbers, electron orbitals, and energy levels. Some key points:
- The quantum mechanical model describes the probable locations of electrons in an atom and how they can have discrete energy levels. It improved upon earlier planetary and Bohr models.
- Electrons occupy specific orbitals and energy levels denoted by quantum numbers like principal and azimuthal quantum numbers.
- Electron configuration notation specifies the distribution of electrons across orbitals based on Aufbau's principle and other rules.
- Transitions between energy levels explain the emission or absorption of photons and thus the colorsSolid state physics
Solid state physicsimtiazalijoono
╠²
The document discusses the fundamental concepts of solid-state physics, focusing on the atomic structure and the differences between conductors, semiconductors, and insulators. It covers the Bohr model, quantum model, and the significance of valence and free electrons in electrical conduction. Additionally, it explains the band gap and the properties of materials used in electronics.TOP ICSE SCHOOLS IN DELHI NCR
TOP ICSE SCHOOLS IN DELHI NCRScholars Learning
╠²
1) The document discusses the structure and constituents of atoms, including the discovery of electrons, protons, and neutrons through experiments.
2) It describes models of the atom including Thomson's "plum pudding" model, Rutherford's nuclear model, Bohr's model incorporating stationary electron orbits, and the quantum mechanical model involving orbitals and energy levels.
3) Key concepts discussed include the dual wave-particle nature of matter and radiation, Planck's quantum theory and photon concept, and the photoelectric effect.Chapter 1_Atomic Structure_PDF_GENERAL CHEMISTRY
Chapter 1_Atomic Structure_PDF_GENERAL CHEMISTRYMinhQuan85
╠²
The document provides an overview of atomic structure, detailing historical developments in atomic theory from Dalton to Schr├Čdinger and discussing key concepts such as atomic models, subatomic particles, quantum numbers, and the Schr├Čdinger wave equation. It outlines significant findings related to atomic particles including electrons, protons, and neutrons, and explains various models including Bohr's model and the modern quantum mechanical model. Additionally, it touches on wave-particle duality and the significance of quantum numbers in determining the state of electrons in an atom.Quantum mechanical model
Quantum mechanical modelIlac Bernardo
╠²
The document outlines the quantum mechanical model of the atom, detailing the concept of quanta and the contributions of physicists like Max Planck, Albert Einstein, and Niels Bohr to our understanding of atomic structure. It explains how electrons occupy quantized energy levels and introduces quantum numbers to describe their positions and behaviors within an atom. Additionally, it highlights significant contributions from key figures in quantum mechanics, including the development of foundational theories such as wave mechanics and the uncertainty principle.Atomic Models.pptx
Atomic Models.pptxArnelButlig
╠²
Democritus first proposed in 400 BC that matter is made of indivisible particles called atoms. This idea was ignored for over 2000 years. In the early 1800s, Dalton proposed that atoms are small, hard spheres that make up elements. Thomson's 1897 discovery of the electron led to his "plum pudding" model of atoms with positive charge and embedded electrons. Rutherford's 1909 gold foil experiment showed that atoms have a small, dense nucleus surrounded by empty space. Bohr's 1913 model depicted electrons orbiting the nucleus in fixed energy levels like planets around the sun. Modern atomic theory describes electrons as existing in probabilistic "clouds" or orbitals around the nucleus based on quantum mechanics.TOP ICSE SCHOOLS IN DELHI NCR
TOP ICSE SCHOOLS IN DELHI NCRScholars Learning
╠²
The document outlines the structure of the atom, detailing the discoveries of electrons, protons, and neutrons, as well as various atomic models including Thomson's, Rutherford's, and Bohr's models. It discusses concepts such as atomic number, mass number, electromagnetic wave theory, and introduces Planck's quantum theory to explain phenomena like the photoelectric effect. The document also covers advanced topics like the Heisenberg uncertainty principle, de Broglie's dual nature of matter, and the quantum mechanical model of the atom, including orbital shapes and energy levels.Quantum theory and the╠²atom
Quantum theory and the╠²atomKamal Metwalli
╠²
This document provides an overview of Bohr's model of the atom and the development of the quantum mechanical model. It discusses key aspects of Bohr's model including discrete energy levels and allowed electron orbits. It then describes limitations of Bohr's model and the proposal of the quantum mechanical model treating electrons as waves. The quantum mechanical model uses wave functions and orbitals to describe electron location probabilities rather than defined orbits. It introduces the concepts of principal and subenergy levels characterized by quantum numbers that specify allowed electron configurations.More from AlvinJuanino (13)
Electron configuration-Grade 9 Science.pptx
Electron configuration-Grade 9 Science.pptxAlvinJuanino
╠²
Electron configuration-Grade 9 Science.pptxRespiratory and circulatory system-Science 9.pptx
Respiratory and circulatory system-Science 9.pptxAlvinJuanino
╠²
Respiratory and circulatory system-Science 9.pptxGENERATING PATTERNS- GRADE 10 MATHEMATICS.pptx
GENERATING PATTERNS- GRADE 10 MATHEMATICS.pptxAlvinJuanino
╠²
GENERATING PATTERNS- GRADE 10 MATHEMATICS.pptxPatterns in the sky -Constellations.pptx
Patterns in the sky -Constellations.pptxAlvinJuanino
╠²
A star is a massive ball of plasma that emits light and can be characterized by brightness, color, surface temperature, size, and mass. Brightness is determined by luminosity and magnitude, while a star's color indicates its surface temperature, with cooler stars appearing red and hotter ones blue. Stars are measured against our sun's size and mass, with notable examples including Rigel, which exceeds the sun's dimensions significantly.Hypertext Markup Language in Grade 8.pptx
Hypertext Markup Language in Grade 8.pptxAlvinJuanino
╠²
The document outlines an activity focused on building a structure using specific materials, aiming to teach HTML concepts including its definition, elements, and historical development. It includes guided questions to promote reflection on teamwork and framework significance. Additionally, it provides a brief history of HTML development, detailing key milestones from its inception to the latest versions.21ST Century Learning in Graduate School.pptx
21ST Century Learning in Graduate School.pptxAlvinJuanino
╠²
The document discusses 21st century learning in science, emphasizing the integration of traditional knowledge with essential skills for addressing global challenges. It highlights the importance of inquiry-based education, problem-solving related to real-world issues, and the use of case studies to foster critical thinking. Overall, the goal is to prepare students to navigate a rapidly changing world through enhanced scientific understanding and problem-solving capabilities.Math Quiz Bee in Grade 10 - Mathematics.pptx
Math Quiz Bee in Grade 10 - Mathematics.pptxAlvinJuanino
╠²
The document presents a series of math quiz questions covering topics such as sequences, arithmetic means, polynomials, angles, and geometry. It includes easy, average, and difficult questions designed to assess mathematical knowledge. Each question is multiple-choice with options provided for selection.When SunŌĆÖs Rays Strike-Grade 9 Science.pptx
When SunŌĆÖs Rays Strike-Grade 9 Science.pptxAlvinJuanino
╠²
The document explains how both latitude and altitude affect climate. It states that areas near the equator have higher temperatures, while temperatures decrease with increased latitude and altitude. Additionally, it highlights that as altitude increases, air temperature drops due to lower air pressure and density.Volcanic Activity Grade 9 science .docx
Volcanic Activity Grade 9 science .docxAlvinJuanino
╠²
The document outlines an educational activity focused on classifying volcanoes in the Philippines as active or inactive. It includes objectives, materials needed, procedures for plotting volcano locations on a map, and guiding questions for analysis. A table with various volcanoes, their coordinates, eruption history, and a legend for classification is also provided.The Different types of Eruption of Volcanoes .ppt
The Different types of Eruption of Volcanoes .pptAlvinJuanino
╠²
The document discusses the classification of volcanoes based on eruption types and cone shapes, detailing three cone shapes: cinder cones, shield cones, and composite cones. It describes six eruption types, ranging from least to most explosive: icelandic, hawaiian, strombolian, vulcanian, pelean, and plinian, highlighting their characteristics and the resulting cone shapes. The most dangerous eruptions, pelean and plinian, are noted for their extreme explosiveness and devastating effects, such as those seen in historic events like the eruptions of Mount St. Helens and Mount Vesuvius.Ad
Recently uploaded (20)
Assisting Individuals and Families to Promote and Maintain Health ŌĆō Unit 7 | ...
Assisting Individuals and Families to Promote and Maintain Health ŌĆō Unit 7 | ...RAKESH SAJJAN
╠²
This PowerPoint presentation is based on Unit 7 ŌĆō Assisting Individuals and Families to Promote and Maintain Their Health, a core topic in Community Health Nursing ŌĆō I for 5th Semester B.Sc Nursing students, as per the Indian Nursing Council (INC) guidelines.
The unit emphasizes the nurseŌĆÖs role in family-centered care, early detection of health problems, health promotion, and appropriate referrals, especially in the context of home visits and community outreach. It also strengthens the studentŌĆÖs understanding of nursing responsibilities in real-life community settings.
¤ōś Key Topics Covered in the Presentation:
Introduction to family health care: needs, principles, and objectives
Assessment of health needs of individuals, families, and groups
Observation and documentation during home visits and field assessments
Identifying risk factors: environmental, behavioral, genetic, and social
Conducting growth and development monitoring in infants and children
Recording and observing:
Milestones of development
Menstrual health and reproductive cycle
Temperature, blood pressure, and vital signs
General physical appearance and personal hygiene
Social assessment: understanding family dynamics, occupation, income, living conditions
Health education and counseling for individuals and families
Guidelines for early detection and referral of communicable and non-communicable diseases
Maintenance of family health records and individual health cards
Assisting families with:
Maternal and child care
Elderly and chronic disease management
Hygiene and nutrition guidance
Utilization of community resources ŌĆō referral linkages, support services, and local health programs
Role of nurse in coordinating care, advocating for vulnerable individuals, and empowering families
Promoting self-care and family participation in disease prevention and health maintenance
This presentation is highly useful for:
Nursing students preparing for internal exams, university theory papers, or community postings
Health educators conducting family teaching sessions
Students conducting fieldwork and project work during community postings
Public health nurses and outreach workers dealing with preventive, promotive, and rehabilitative care
ItŌĆÖs structured in a step-by-step format, featuring tables, case examples, and simplified explanations tailored for easy understanding and classroom delivery.Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecdrazelitouali
╠²
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition Oecd
Energy Balances Of Oecd Countries 2011 Iea Statistics 1st Edition OecdPlate Tectonic Boundaries and Continental Drift Theory
Plate Tectonic Boundaries and Continental Drift TheoryMarie
╠²
This 28 slide presentation covers the basics of plate tectonics and continental drift theory. It is an effective introduction into a full plate tectonics unit study, but does not cover faults, stress, seismic waves, or seafloor spreading.
To download PDF, visit The Homeschool Daily. We will be uploading more slideshows to follow this one. Blessings, Marie Introduction to Generative AI and Copilot.pdf
Introduction to Generative AI and Copilot.pdfTechSoup
╠²
In this engaging and insightful two-part webinar series, where we will dive into the essentials of generative AI, address key AI concerns, and demonstrate how nonprofits can benefit from using MicrosoftŌĆÖs AI assistant, Copilot, to achieve their goals.
This event series to help nonprofits obtain Copilot skills is made possible by generous support from Microsoft.Capitol Doctoral Presentation -June 2025.pptx
Capitol Doctoral Presentation -June 2025.pptxCapitolTechU
╠²
║▌║▌▀Żs from a Capitol Technology University presentation covering doctoral programs offered by the university. All programs are online, and regionally accredited. The presentation covers degree program details, tuition, financial aid and the application process.Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...
Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...Rajdeep Bavaliya
╠²
Get ready to embark on a cosmic quest as we unpack the archetypal power behind Christopher NolanŌĆÖs ŌĆśInterstellar.ŌĆÖ Discover how heroŌĆÖs journey tropes, mythic symbols like wormholes and tesseracts, and themes of love, sacrifice, and environmental urgency shape this epic odyssey. Whether youŌĆÖre a film theory buff or a casual viewer, youŌĆÖll learn why CooperŌĆÖs journey resonates with timeless mythsŌĆöand what it means for our own future. Smash that like button, and follow for more deep dives into cinemaŌĆÖs greatest stories!
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 109: Literary Theory & Criticism and Indian Aesthetics
Submitted Date: April 5, 2025
Paper Name: Literary Theory & Criticism and Indian Aesthetics
Topic: Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes in NolanŌĆÖs Cosmic Odyssey
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/vHLaLZPHumk
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/archetypal-journeys-in-interstellar-exploring-universal-themes-in-nolan-s-cosmic-odyssey.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#ChristopherNolan #Interstellar #NolanFilms #HeroJourney #CosmicOdyssey #FilmTheory #ArchetypalCriticism #SciFiCinema #TimeDilation #EnvironmentalCinema #MythicStorytelling
Keyword Tags:
Interstellar analysis, Christopher Nolan archetypes, heroŌĆÖs journey explained, wormhole symbolism, tesseract meaning, myth in sci-fi, cinematic archetypes, environmental themes film, love across time, Nolan film breakdownGEOGRAPHY-Study Material [ Class 10th] .pdf
GEOGRAPHY-Study Material [ Class 10th] .pdfSHERAZ AHMAD LONE
╠²
"Geography Study Material for Class 10th" provides a comprehensive and easy-to-understand resource for key topics like Resources & Development, Water Resources, Agriculture, Minerals & Energy, Manufacturing Industries, and Lifelines of the National Economy. Designed as per the latest NCERT/JKBOSE syllabus, it includes notes, maps, diagrams, and MODEL question Paper to help students excel in exams. Whether revising for exams or strengthening conceptual clarity, this material ensures effective learning and high scores. Perfect for last-minute revisions and structured study sessions.Overview of Employee in Odoo 18 - Odoo ║▌║▌▀Żs
Overview of Employee in Odoo 18 - Odoo ║▌║▌▀ŻsCeline George
╠²
The employee module is a core component of the HR workspace that helps the business to get the employee activities and details. This would also allow you to get the employee details by acting as a centralized system and accessing, updating, and managing all the other employee data. Non-Communicable Diseases and National Health Programs ŌĆō Unit 10 | B.Sc Nursi...
Non-Communicable Diseases and National Health Programs ŌĆō Unit 10 | B.Sc Nursi...RAKESH SAJJAN
╠²
This PowerPoint presentation is prepared for Unit 10 ŌĆō Non-Communicable Diseases and National Health Programs, as per the 5th Semester B.Sc Nursing syllabus outlined by the Indian Nursing Council (INC) under the subject Community Health Nursing ŌĆō I.
This unit focuses on equipping students with knowledge of the causes, prevention, and control of non-communicable diseases (NCDs), which are a major public health challenge in India. The presentation emphasizes the nurseŌĆÖs role in early detection, screening, management, and referral services under national-level programs.
¤ö╣ Key Topics Included:
Definition, burden, and impact of NCDs in India
Epidemiology, risk factors, signs/symptoms, prevention, and management of:
Diabetes Mellitus
Hypertension
Cardiovascular Diseases
Stroke & Obesity
Thyroid Disorders
Blindness
Deafness
Injuries and Accidents (incl. road traffic injuries and trauma guidelines)
NCD-2 Cancers:
Breast Cancer
Cervical Cancer
Oral Cancer
Risk factors, screening, diagnosis, early signs, referral & palliative care
Role of nurse in screening, referral, counseling, and continuum of care
National Programs:
National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS)
National Program for Control of Blindness
National Program for Prevention and Control of Deafness
National Tobacco Control Program (NTCP)
Introduction to Universal Health Coverage and Ayushman Bharat
Use of standard treatment protocols and referral flowcharts
This presentation is ideal for:
Classroom lectures, field assignments, health education planning, and student projects
Preparing for university exams, class tests, and community field postingsHow to Manage Multi Language for Invoice in Odoo 18
How to Manage Multi Language for Invoice in Odoo 18Celine George
╠²
Odoo supports multi-language functionality for invoices, allowing you to generate invoices in your customersŌĆÖ preferred languages. Multi-language support for invoices is crucial for businesses operating in global markets or dealing with customers from different linguistic backgrounds. Measuring, learning and applying multiplication facts.
Measuring, learning and applying multiplication facts.cgilmore6
╠²
║▌║▌▀Żs from a presentation by Professor Camilla Gilmore to the Association of Teachers of Mathematics and Mathematics Association Primary Interest group in June 2025.
This gave an overview of two studies that investigated children's multiplication fact knowledge. These studies were part of the SUM research project based at the University of Nottingham and Loughborough University. For more information see www.sumproject.org.ukTHERAPEUTIC COMMUNICATION included definition, characteristics, nurse patient...
THERAPEUTIC COMMUNICATION included definition, characteristics, nurse patient...parmarjuli1412
╠²
The document provides an overview of therapeutic communication, emphasizing its importance in nursing to address patient needs and establish effective relationships. THERAPEUTIC COMMUNICATION included some topics like introduction of COMMUNICATION, definition, types, process of communication, definition therapeutic communication, goal, techniques of therapeutic communication, non-therapeutic communication, few ways to improved therapeutic communication, characteristics of therapeutic communication, barrier of THERAPEUTIC RELATIONSHIP, introduction of interpersonal relationship, types of IPR, elements/ dynamics of IPR, introduction of therapeutic nurse patient relationship, definition, purpose, elements/characteristics , and phases of therapeutic communication, definition of Johari window, uses, what actually model represent and its areas, THERAPEUTIC IMPASSES and its management in 5th semester Bsc. nursing and 2nd GNM students2025 June Year 9 Presentation: Subject selection.pptx
2025 June Year 9 Presentation: Subject selection.pptxmansk2
╠²
2025 June Year 9 Presentation: Subject selectionBINARY files CSV files JSON files with example.pptx
BINARY files CSV files JSON files with example.pptxRamakrishna Reddy Bijjam
╠²
BINARY FILES, CSV FILESFIRST DAY HIGH orientation for mapeh subject in grade 10.pptx
FIRST DAY HIGH orientation for mapeh subject in grade 10.pptxGlysdiEelesor1
╠²
basic orientation of the first day highjune 10 2025 ppt for madden on art science is over.pptx
june 10 2025 ppt for madden on art science is over.pptxroger malina
╠²
art science is over -talk by roger malina for jack madden groupPaper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...
Paper 109 | Archetypal Journeys in ŌĆśInterstellarŌĆÖ: Exploring Universal Themes...Rajdeep Bavaliya
╠²
Ad
QUANTUM MECHANICAL MODEL OF THE ATOM.pptx
- 1. QUANTUM MECHANICAL MODEL OFTHE ATOM
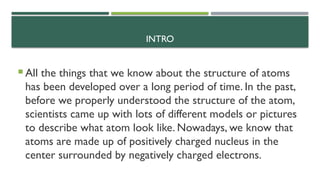
- 2. INTRO ’éĪ All the things that we know about the structure of atoms has been developed over a long period of time. In the past, before we properly understood the structure of the atom, scientists came up with lots of different models or pictures to describe what atom look like. Nowadays, we know that atoms are made up of positively charged nucleus in the center surrounded by negatively charged electrons.
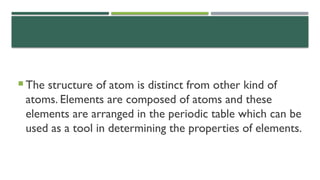
- 4. ’éĪ The structure of atom is distinct from other kind of atoms. Elements are composed of atoms and these elements are arranged in the periodic table which can be used as a tool in determining the properties of elements.
- 5. FORDA HULA ANG FERSON!
- 6. FORDA HULA ANG FERSON!
- 7. FORDA HULA ANG FERSON!
- 8. FORDA HULA ANG FERSON!
- 9. FORDA HULA ANG FERSON!
- 10. FORDA HULA ANG FERSON!
- 11. QUESTIONS TO ANSWER ’éĪ What is Quantum Mechanical Model? ’éĪ Who proposed the Quantum Mechanical Model of an Atom? ’éĪ What is the inter-relationship between principal energy level, sublevel, orbital and electron? ’éĪ Do you know how to write the electron configuration of elements?
- 12. THREE PHYSICISTS LEDTHE DEVELOPMENT OF A BETTER MODEL OF AN ATOM KNOWN AS QUANTUM MECHANICAL MODEL. ’éĪ Louie de Broglie ’éĪ Erwin Schrodinger ’éĪ Werner Karl Heisenberg.
- 13. THREE PHYSICISTS LEDTHE DEVELOPMENT OF A BETTER MODEL OF AN ATOM KNOWN AS QUANTUM MECHANICAL MODEL. ’éĪ De Broglie proposed that the electron (which is thought of as a particle) could also be thought of as a wave.
- 14. THREE PHYSICISTS LEDTHE DEVELOPMENT OF A BETTER MODEL OF AN ATOM KNOWN AS QUANTUM MECHANICAL MODEL. ’éĪ Schrodinger used this idea to develop a mathematical equation to describe the hydrogen atom.
- 15. THREE PHYSICISTS LEDTHE DEVELOPMENT OF A BETTER MODEL OF AN ATOM KNOWN AS QUANTUM MECHANICAL MODEL. ’éĪ Heisenberg discovered that for a very small particle like the electron, its location cannot be exactly known and how it is moving.This is called uncertainty principle.
- 16. Instead, these scientists believed that there is only a probability that the electron can be found in a certain volume in space around the nucleus.This volume or region of space around the nucleus where the electron is most likely to be found is called an atomic orbital.Thus, we could only guess the most probable location of the electron at a certain time to be within a certain volume of space surrounding the nucleus.
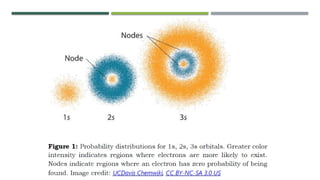
- 17. The quantum mechanical model of the atom comes from the mathematical solution to the Schrodinger equation.The quantum mechanical model views an electron as a cloud of negative charge having a certain geometrical shape.This model shows how likely an electron could be found in various locations around the nucleus. However, the model does not give any information about how the electron moves from one position to another.
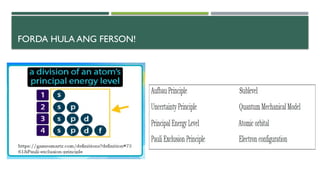
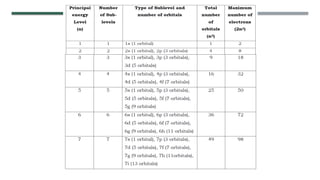
- 19. ’éĪ The picture shows that the greater color intensity an area has, the greater is the probability of finding electrons in that area. ’éĪ The quantum mechanical model also gives information about the energy of the electron.The model also describes the region of space around the nucleus as consisting of shells. ’éĪ These shells are also called principal or main energy levels. The principal energy levels or shells may have one or more sublevels. ’éĪ These sublevels are assigned with letters: s, p, d, f, g, h and i
- 21. The principal quantum number is always equal to the number of sub-levels within that principal energy level. That is, the principal energy level 1 will have 1 sub-level, principal energy level 2 will have 2 sub-levels, principal energy level 3 will have 3 sub-levels, and so on. The maximum number of electrons that can occupy a principal energy level is given by the formula 2, where n is the principal quantum number.
- 22. The maximum number of electrons that can occupy the first principal energy level is 2 ((2) ). For energy level 2, the maximum number of electrons is 8 ((2)), and for the third principal energy level, the maximum number of electrons is 18 ((2)).
- 23. The largest known element contains 118 electrons. Quantum mechanics sets no limit as to how many energy levels exist, but no more than 7 principal energy levels are needed to describe the electrons of all known atoms.