R and S Nomenclature for Stereoisomers.pptx
- 1. R and S Nomenclature for Stereoisomers
- 2. ŌĆó Stereochemistry is the study of the relative arrangement of atoms or groups in a molecule in three dimensional space. ŌĆó Stereo-isomers are molecules, which have the same chemical formula and bond connectivity but different relative arrangement in three-dimensional space. WHAT IS STEREOCHEMISTRY
- 4. ŌĆó Enantiomers: Nonsuperimposable mirror images, different molecules with different properties. STEREOISOMERS
- 5. ŌĆó Cis-1,2-dichlorocyclohexane is achiral because the molecule has an internal plane of symmetry. Both structures above can be superimposed. ŌĆó Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the images are nonsuperimposable and the molecule will have two enantiomers. Cis and Trans Cyclic Compounds
- 6. ŌĆó Different molecules (enantiomers) must have different names. ŌĆó Usually only one enantiomer will be biologically active. ŌĆó Configuration around the chiral carbon is specified with (R) and (S). (R) and (S) Nomenclature
- 7. ŌĆó CahnŌĆōIngoldŌĆōPrelog Rules ŌĆó Assign a priority number to each group attached to the chiral carbon. ŌĆó Priority is assigned according to atomic number. The highest atomic number assigned is the highest priority #1. ŌĆó In case of ties, look at the next atoms along the chain. ŌĆó Double and triple bonds are treated like bonds to duplicate atoms. (R) and (S) Nomenclature
- 8. ŌĆó Once priorities have been assigned, the lowest priority group (#4) should be moved to the back if necessary. ŌĆó Atomic number: F > N > C > H Assign Priorities
- 9. ŌĆó Draw an arrow from Group 1 to Group 2 to Group 3 and back to Group 1. Ignore Group 4. ŌĆó Clockwise = (R) and Counterclockwise = (S) Assign Priorities Counterclockwise (S)
- 10. ŌĆó When rotating to put the lowest priority group in the back, keep one group in place and rotate the other three. Example C OH CH3CH2CH2 H CH2CH3 1 2 3 4 C CH2CH3 CH3CH2CH2 OH H 1 2 3 4 rotate Clockwise (R)
- 11. ŌĆó Same boiling point, melting point, and density. ŌĆó Same refractive index. ŌĆó Rotate the plane of polarized light in the same magnitude, but in opposite directions. ŌĆó Different interaction with other chiral molecules: ŌĆó Active site of enzymes is selective for a specific enantiomer. ŌĆó Taste buds and scent receptors are also chiral. Enantiomers may have different smells. Properties of Enantiomers
- 12. ŌĆó Enantiomers rotate the plane of polarized light in opposite directions, but same number of degrees. Optical Activity
- 13. Polarimeter Clockwise Dextrorotatory (+) Counterclockwise Levorotatory (-) Not related to (R) and (S)
- 14. ŌĆó Observed rotation depends on the length of the cell and concentration, as well as the strength of optical activity, temperature, and wavelength of light. ŌĆó Where ’üĪ (observed) is the rotation observed in the polarimeter, c is concentration in g/mL and l is length of sample cell in decimeters. Specific Rotation [’üĪ] = ’üĪ (observed) c ’éĘ l
- 15. ŌĆó Equal quantities of d- and l- enantiomers. ŌĆó Notation: (d,l) or (’é▒) ŌĆó No optical activity. ŌĆó The mixture may have different boiling point (b. p.) and melting point (m. p.) from the enantiomers!. Racemic Mixtures
- 16. ŌĆó Equal quantities of d- and l- enantiomers. ŌĆó Notation: (d,l) or (’é▒) ŌĆó No optical activity. ŌĆó The mixture may have different boiling point (b. p.) and melting point (m. p.) from the enantiomers!. Racemic Mixtures
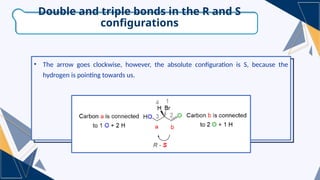
- 17. ŌĆó Bromine is the priority and the hydrogen is number four. Carbon ŌĆ£aŌĆØ is connected to one oxygen and two hydrogens. Carbon ŌĆ£bŌĆØ is connected to one oxygen and one hydrogen. ŌĆó However, because of the double bond, carbon ŌĆ£bŌĆØ is treated as if it is connected to two oxygens. The same rule is applied for any other double or triple bond. So, when you see a double bond count it as two single bonds when you see a triple bond cut it as three single bonds. Double and triple bonds in the R and S configurations
- 18. ŌĆó The arrow goes clockwise, however, the absolute configuration is S, because the hydrogen is pointing towards us. Double and triple bonds in the R and S configurations
- 19. ŌĆó A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Mesomers are compounds with zero net rotation of plane polarised light. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. ŌĆó Meso compounds feature several chiral centres and are achiral substances. Despite having stereocenters, it is overlaid on its mirror counterpart and is optically inactive. Meso compounds
- 20. ŌĆó Meso compounds feature chiral centres (sp3 hybridised tetrahedral atoms bound to four distinct groups), yet they are achiral generally due to symmetry. ŌĆó As mentioned, a meso compound should include at least two chiral s p 3 hybridised atoms with the exception of the nitrogen atom of a tertiary amine and at least one internal plane that splits the molecule into two mirror copies. Meso compounds














![ŌĆó Observed rotation depends on the length of the cell and concentration, as well
as the strength of optical activity, temperature, and wavelength of light.
ŌĆó Where ’üĪ (observed) is the rotation observed in the polarimeter, c is
concentration in g/mL and l is length of sample cell in decimeters.
Specific Rotation
[’üĪ] = ’üĪ
(observed)
c ’éĘ l](https://image.slidesharecdn.com/randsnomenclatureforstereoisomers-241119222336-ff087181/85/R-and-S-Nomenclature-for-Stereoisomers-pptx-14-320.jpg)