Radiological evaluation aasld 2011
- 1. AASLD 2011 BLINDED INDEPENDENT CENTRAL RESPONSE ASSESSMENT USING RECIST, MODIFIED RECIST, AND CHOI CRITERIA IN PATIENTS TREATED WITH SORAFENIB FOR ADVANCED HEPATOCELLULAR CARCINOMA Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, B¨¦atrice Larroque, Laurent Castera, Chantal Dreyer, Val¨¦rie Vilgrain, Jacques Belghiti, Eric Raymond, Sandrine Faivre Beaujon University Hospital Clichy, France
- 2. AASLD 2011 No disclosure to declare
- 3. RECIST do Not Capture the True Benefit of Sorafenib in HCC ? Sorafenib improves survival but yields low objective response rate by RECIST (< 5%)1,2 ? Sustained survival despite NO response by RECIST suggest that RECIST are inappropriate to capture the true benefit of sorafenib3 ? Modified RECIST (mRECIST)4 are used to assess vascular effects of TACE5 and CHOI criteria were proposed to evaluate necrosis induced by targeted agents in HCC6 1 LlovetJM ; N Engl J Med 2008 4 Lencioni R ; Semin Liver Dis. 2010 2 Cheng AL ; Lancet Oncol 2009 5 Gillmore R, J Hepatol 2011 3 Edeline J, Cancer 2011 6 Faivre S, Clin Cancer Res 2011 AASLD 2011
- 4. Objectives ? At the first tumor evaluation, are mRECIST and CHOI criteria predicting overall survival in patients with HCC treated with sorafenib? ? Can mRECIST and CHOI criteria reallocate to the objective response group, patients who were inappropriately considered non-responders by RECIST? AASLD 2011
- 5. Study Method/Radiological Evaluation ? Retrospective single center cohort analysis ? January 2007- December 2009 ? Beaujon Hospital ? Baseline evaluation within 6 weeks prior to sorafenib ? First tumor evaluation by CT-scan 2-3 months after sorafenib initiation AASLD 2011
- 6. Radiological Evaluation ? Quality control criteria ¨C Multiphasic CT-scan fully available for central review ¨C Central Review of data by a radiologist highly experienced in liver cancers, blinded to clinical data ¨C Evaluation of tumor response by RECIST, mRECIST, and CHOI criteria RECIST mRECIST CHOI criteria HU AASLD 2011
- 7. Results: Study Population Selection Patients with BCLC B-C, Child-Pugh A-B, advanced hepatocellular carcinoma treated with sorafenib from 2007 to 2009 (n=82) Non-evaluable patients (n=22) ¨C Non-evaluable CT scan (n=9) ¨C Target lesions in pretreated area (n=9) ¨C No target lesion (n=3) ¨C Missing data (n=4) ? Lost of follow up ? Early death Patients evaluated in this study (n=60) AASLD 2011
- 8. Patient Characteristics Median age, years 61 (37-77) Sex M/F 52/8 Etiology, % (number of patients) Viral 48 (29) Alcohol 23 (14) Child Pugh Score, % (number of patients) A 80 (48) B 20 (12) BCLC stage , % (number of patients) B 33 (20) C 67 (40) Pathological diagnosis, % (number of patients) 88 (53) Extrahepatic Spread , % (number of patients) 35 (21) Prior treatments, % (number of patients) None 32 (19) Surgery 27 (16) Radio Frequency Ablation 5 (3) Trans-Arterial Chemo-Embolization 36 (22) Median duration of sorafenib, months 5.7 Median time for the first evaluation, months 2.1 AASLD 2011
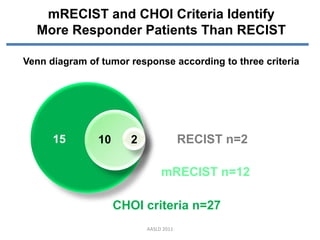
- 9. mRECIST and CHOI Criteria Identify More Responder Patients Than RECIST Venn diagram of tumor response according to three criteria 15 10 2 RECIST n=2 mRECIST n=12 CHOI criteria n=27 AASLD 2011
- 10. Response Rates by RECIST, mRECIST, and CHOI Criteria 70 Objective response % of response rates by each criteria 60 Stable Disease 62 Progressive Disease 50 40 48 45 30 35 32 30 20 23 21 10 3 0 RECIST mRECIST CHOI N=60 N=56* AASLD 2011 N=60 *4 pts non evaluable
- 11. At the first tumor evaluation, are mRECIST and CHOI criteria predicting overall survival in patients with HCC treated with sorafenib? AASLD 2011
- 12. Responses by RECIST Criteria Correlate with Survival 1 0,9 0,8 RECIST Probability of survival 0,7 0,6 Objective response (PR/CR) 0,5 Stable Disease Progressive Disease 0,4 0,3 0,2 0,1 p=0.0012 0 0 5 10 15 20 25 30 35 40 45 Duration of survival, months
- 14. Can mRECIST and CHOI criteria reallocate to the objective response group, patients who were inappropriately considered non- responders by RECIST? AASLD 2011
- 15. Examples of discrepancies Between methods of evaluation RECIST mRECIST CHOI criteria Baseline ? Stable ? Response ? Response ¨K HU Baseline ? Progression ? Response ? Response ¨K HU AASLD 2011
- 16. Many Stable and Some Progressive Diseases by RECIST Are Objective Responses by mRECIST and CHOI Criteria Objective responses according to each criteria 45% Number of patients with objective response 30 3 25 Response by RECIST Progressive Disease 20 21.4% 15 22 Stable Disease 1 Objective response 10 3.3% 9 5 2 2 2 0 Responders Responders Responders RECIST (n=2) AASLD 2011 (n=27) mRECIST (n=12) CHOI
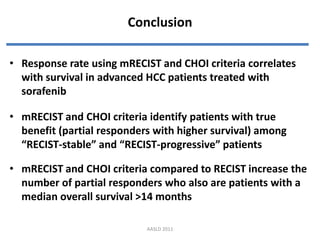
- 17. Conclusion ? Response rate using mRECIST and CHOI criteria correlates with survival in advanced HCC patients treated with sorafenib ? mRECIST and CHOI criteria identify patients with true benefit (partial responders with higher survival) among ¡°RECIST-stable¡± and ¡°RECIST-progressive¡± patients ? mRECIST and CHOI criteria compared to RECIST increase the number of partial responders who also are patients with a median overall survival >14 months AASLD 2011