Rapid Microbiological Methods in the Pharmaceutical Industry
- 1. Rapid Microbiological Methods in the Pharmaceutical Industry Rania Hussein Smerat
- 2. Introduction • Microbial contamination poses a significant challenge in the pharmaceutical industry, where ensuring the safety and efficacy of products is Crucial. • For example, certain microorganisms may affect the packaging and/or therapeutic activity of the product, rendering it inactive, unstable, unsafe, or unable to deliver its intended dose and treatment. More importantly, the presence of an opportunistic pathogen or objectionable microorganism could result in a significant clinical event, such as an infection or death. • Pharmaceutical manufacturers are expected to demonstrate their products are free of microorganisms that could cause harm either to the product or to the patient or consumer. • Microbiological testing is key to the operation of pharmaceutical facilities. This testing is used to assess the microbiological quality and attributes of the product, components (e.g. active pharmaceutical ingredients, excipients, and water), manufacturing environment, equipment, processes and the utilities.
- 3. Accordingly, these assessments should effectively determine the numbers of viable microorganisms in each test sample and reveal the presence (or absence) of objectionable microorganisms. The conventional microbiological methods used are limited by the time it takes to grow the microorganism under the specifed test conditions. These methods have traditionally required days until the results are obtained. We want methods that are: Faster > More Sensitive > Lower Price > Higher Throughput > More Specific > New Targets The new methods offer many advantages, including shorter processing times, improved accuracy, enhanced limits of detection, and other key attributes associated with the method. Specific concerns: • Validations • regulatory acceptance by (MHRA) and (FDA) • false-positive or false-negative
- 4. OVERVIEW OF MICROBIOLOGICAL METHODS There are three basic types of microbiological evaluations conducted: Presence/Absence Tests simply determines whether a microorganism is present in a sample. Enumeration Tests to determine the number of microorganisms present. Identification Methods if an organism is present what organism is it.
- 5. Traditional techniques most commonly used are: - Pour plates - Spread plating - Drop counts (Miles Misra) - Most probable number (MPN) - Direct or microscopic counts - Membrane filtration
- 6. Rapid Microbiological Methods Rapid Microbial Methods (RMM) offer significant advantages over traditional growth- based methods. They are more sensitive, accurate, precise, and robust. Many RMM systems are fully automated, capable of higher sample throughput, continuous data collection, real-time data acquisition, and trend analysis. Additionally, they significantly reduce the time required to obtain results, delivering them in hours or minutes instead of days or weeks. Most rapid microbial methods (RMM) can detect a wide variety of microorganisms (e.g., bacteria, yeast, and mold) or target specific species. They can enumerate the number microorganisms in a sample and identify them at the genus, species, subspecies, or strain level. The detection, quantification, and identification depend on the specific technology, analytical method, instrumentation, and software used.
- 7. TYPES OF NEW TECHNOLOGIES—RAPID MICROBIOLOGICAL METHODS: There are also methods that incorporate more than one type of technology into the system, e.g., may be able to detect, enumerate, and identify microorganisms or addition of a viability assessment to a system that uses another technology like spectroscopy to detect microorganisms. An example is Raman spectroscopy. Growth-based methods Viability-based methods Cellular component or artifact-based technologies Nucleic acid-based Spectroscopic methods The choice of the most suitable rapid method depends on factors such as the type of analysis (qualitative, quantitative, or identification), application scope, required sensitivity and specificity, microorganism range, sample type and quantity, time-to-result, sample size, and compatibility with test preparation and handling.
- 8. Growth-Based RMMs Systems in this category assume that organism detection is dependent upon measurement of attributes that require growth of the microorganisms. Rapid methods that rely on microbial growth in conventional liquid or solid media are used for various applications, such as bioburden testing, presence/absence testing, environmental monitoring (EM), water analysis, and microbial identification. These systems quickly detect growing organisms or enumerate microcolonies using specialized scientific principles, including: 1. Measuring changes in electrical conductivity or impedance. • This technique is based on the fact that microbial growth breaks down larger, relatively uncharged molecules (e.g., proteins, sugars) into smaller, highly charged molecules (e.g., amino acids, fatty acids, and lactic acid). • Growth is detected by monitoring ion movement between electrodes (conductance) or charge storage at the electrode surface (capacitance). • Impedance systems can detect measurable electrical changes in liquid media caused by microbial growth, even before visible turbidity appears. • A modern example of this technology is the Sy-Lab BacTrac system. • These systems do not provide direct quantitative results (CFU).
- 9. 2. Detecting respiratory byproducts (e.g., oxygen consumption or carbon dioxide production). Microorganisms grown in liquid culture produce carbon dioxide as a byproduct. In a closed container, microbial growth can be detected by monitoring changes in the carbon dioxide levels. • As microorganisms grow, they release carbon dioxide. • This carbon dioxide diffuses into a liquid emulsion sensor within the system. • The sensor undergoes a color change, signaling the presence of microorganisms. Examples of Technologies: 1. BioMérieux’s BacT/ALERT 2. Becton Dickinson (BD) Diagnostic Systems BACTEC FX • Both methods are qualitative. • Effective and rapid detection of microbial presence, especially in closed systems. BACT/ALERT
- 10. 3. Utilizing carbohydrates and other substrates. Some identification technologies determine the ability of microorganisms to metabolize biochemical and carbohydrate substrates, often using a microtiter plate format. These systems detect microbial growth by monitoring changes in kinetic reactions or turbidity. Microtiter Plate Format: • Each well contains a dehydrated substrate (e.g., carbon sources). • Microorganisms metabolize the substrates, leading to observable changes. Examples of Technologies: • Biolog Omnilog System: - Includes tetrazolium violet dye in the wells with the carbon source. - If a microorganism utilizes the carbon source, the well turns purple. • bioMérieux VITEK 2: - Uses an optical system that monitors wells using visible light wavelengths. - Detects turbidity (growth) or color changes due to substrate metabolism. #Data Analysis: - Both systems generate data in the form of positive or negative responses for each substrate. - This data is compared with an internal database to identify the microorganism. • Requires pure cultures (isolated colonies or pure liquid media cultures). Mixed cultures can result in incorrect identifications or invalid responses.
- 11. 4. Sensing autofluorescence of microcolonies after excitation by specific wavelengths of light 1. Growth Direct (Rapid Micro Biosystems): Uses digital imaging to detect microbial growth on agar by sensing fluorescent signals from microcolonies. It’s a quantitative method that provides cell counts quickly, useful for bioburden studies. Requires filterable samples. 2. EviSight Compact (bioMérieux): Uses high-magnification imaging to count microcolonies on Petri plates every 30 minutes. It’s a nondestructive, automated method for bioburden testing. 3. Biolumix and Soleris Neogen Systems: Detect microbial growth in broth media using dyes that change color or fluorescence. 4. Bactest Speedy Breedy: A portable respirometer that detects pressure changes due to microbial respiration. It’s qualitative and doesn’t provide a cell count. These rapid microbial detection technologies use advanced methods such as digital imaging, growth analysis, and the detection of color or fluorescence changes. Each technology has unique principles of operation and is used to detect microbial growth in a shorter time compared to traditional methods.
- 12. Cellular Component-Based Technologies Cellular component-based RMMs detect specific cellular components or the use of probes that are specific for microbial target sites of interest. For example, adenosine triphosphate (ATP), fatty acids, surface macromolecules, bacterial endotoxin, proteins, and nucleic acids have been used as targets for RMMs for the detection, quantification, and identification of microorganisms. 1. ATP Bioluminescence: • Utilizes ATP as an indicator of microbial viability. In the presence of luciferase enzyme and D- luciferin, ATP reacts to produce light, which is measured using a luminometer. • Quantitative Systems: Provide precise microbial counts (e.g., Millipore Milliflex Rapid system). • Qualitative Systems: Indicate the presence of microorganisms based on light intensity (e.g., Celsis Advance II). • For samples with low microbial levels, an enrichment step (growth in liquid or agar) may be needed to increase detectable ATP levels.
- 13. 2. Fatty Acid Analysis (MIDI Sherlock MIS System): • Analyzes unique fatty acid profiles in microbial cell membranes using gas chromatography. • Identifies bacteria and fungi by comparing fatty acid peaks with a reference database. • Requires isolated colonies or pure cultures for accurate results. 3. Matrix-assisted laser desorption ionization, time of flight (MALDI-TOF) mass spectrometry: • Exposes microorganisms to a laser, generating charged ions that are separated based on their mass-to-charge ratio. The resulting molecular patterns (mass spectra) are matched against a database to identify microbial species. • Identifies bacteria, yeast, mold, and mycobacteria. • Examples include the Bruker MALDI Biotyper and bioMérieux Vitek MS.
- 14. Viability-Based Technologies Viability-based technologies differentiate viable cells from dead cells and can target specific microorganisms using nucleic acid, enzymatic, or monoclonal antibody probes. In many cases, direct labeling of single cells is possible with no cell growth requirement, facilitating time to result in hours or even minutes. • Detect stressed, dormant, or non-culturable cells that traditional methods might miss. • Broad applications include bioburden testing, water analysis, and environmental monitoring. Techniques: 1. Flow Cytometry: - Labels cells with viability markers and passes them through a laser beam for detection (essentially one cell at a time) . - Provides quick quantitative results (in minutes), though precision is limited to 10–50 viable cells. Examples: BD FACSMicroCount, bioMérieux D-Count and BactiFlow.
- 15. 2. Solid-Phase Cytometry: - Captures cells on a filter, stains them with a viability substrate, and uses laser scanning for enumeration. - Can provide highly accurate and precise single-cell counts. - Limited to filterable samples with minimal interference risk. Examples: bioMérieux ScaRDI, LumiByte BV MuScan. • Only viable cells process the substrate to release a fluorescent signal. • A laser scan detects and quantifies labeled microorganisms in 10– 90 minutes. • Specific test kits are available for pathogens like Salmonella, Legionella, E. coli, S. aureus, and fungal spores.
- 16. Nucleic Acid Amplification-Based Technologies Nucleic acid amplification-based technologies use nucleic acid amplification, like PCR, TMA, 16S rRNA typing, or gene sequencing, to detect microorganisms or identify them at various levels (genus, species, strain). 1. PCR (Polymerase Chain Reaction): • Most nucleic acid amplification technologies use PCR as their core scientific method. • PCR employs primers (small DNA fragments) to target and amplify specific DNA sequences. -Detection Techniques: • Fluorescence detection through melting curves: - DNA is heated until it separate, releasing fluorescent dye and lowering the signal. - Melting curves help identify specific target organisms based on their unique fluorescence patterns. Examples of PCR-Based Systems: • Hygiena BAX System: Detects pathogens like Salmonella, L. monocytogenes, E. coli O157:H7, and more. • GeneDisc by Pall Corporation: Detects microorganisms like E. coli, Salmonella, S. aureus, C. albicans, and others. - False Positives: Residual or environmental DNA (even from nonviable cells) can lead to positive results, even when the organism is absent. - PCR results can be obtained within a few hours, making it a rapid diagnostic tool compared to traditional methods.
- 17. 2.TMA (Transcription-Mediated Amplification): • Amplifies RNA instead of DNA, reducing false positives from nonviable cells. • Faster than PCR and requires only a single temperature for amplification. 3. Gene Sequencing: • Uses 16S rDNA to classify microorganisms. • Sequences DNA fragments and compares them to databases for identification. • Common system: Applied Biosystems MicroSEQ.
- 18. Spectroscopy-Based Technologies Optical spectroscopy is an analytical tool that measures the interactions between light and the material being studied. • Light scattering occurs when light’s path is disrupted by particles. • Example: Mie scattering, where scattered light intensity is proportional to particle size. 2. Applications of Mie Scattering: • Mie scattering is widely used in particle counters to detect, count and size of particle (viable cells. in air and water samples). Particles in the range of 0.5–20 µm are analyzed. A UV laser excites biological materials (e.g., NADH, riboflavin, dipicolinic acid), causing them to auto-fluoresce. - No need for reagents, staining, labeling, or culturing. • Examples: Air Sampling Systems: Bio Vigilant IMD-A, Particle Measuring Systems BioLaz, TSI Bio Trak. - These systems can analyze up to 1 m³ of air in 35 minutes. - Ideal for real-time monitoring in environments like non-sterile manufacturing. - They reduce resource usage for labor-intensive evaluations. False Positives: Technologies may detect dead cells, mistaking them for viable organisms.
- 20. Raman Spectroscopy A technique based on light scattering that provides information about molecular vibrations and rotations. • Each microorganism has a unique Raman spectrum, which acts as a fingerprint for identification. • Most Raman systems detect both viable and nonviable cells, this can lead to false positives, particularly in industries where viability confirmation is critical (e.g., non-sterile pharmaceutical manufacturing). • Dead cells may be mistakenly identified as viable, potentially leading to incorrect conclusions about contamination. A viability-staining step can be used before Raman spectroscopy to ensure only viable cells are analyzed,(the mibiC GRAMRAY system incorporates this step) . - Enables simultaneous detection, enumeration, and identification of microorganisms within minutes. • The resulting spectral signatures are matched with a library of known microorganisms for identification.
- 21. Validating Rapid Microbiological Methods Validation is the act of demonstrating and documenting that a procedure operates effectively and repeatedly. Because rapid methods are considered alternatives to existing or compendial microbiology methods, the end user is responsible for demonstrating the rapid method is at least equivalent to, or is non-inferior to, the method intended to be replaced. The validation plan should provide a roadmap for all of the activities that will be required to demonstrate that the rapid method system is suitable for its intended use. There are a number of validation documents that provide guidance on method development, method suitability testing, equivalency studies, the use of statistics, feasibility or proof-of- concept studies, training, technology transfer, and the establishment of validation plans. The three most commonly used guidance documents are as follows: • PDA Technical Report #33, Evaluation, Validation and Implementation of Alternative and Rapid Microbiological Methods (PDA 2013) • USP Chapter < 1223>, Validation of Alternative Microbiological Methods • Ph. Eur. chapter 5.1.6, Alternative Methods for Control of Microbiological Quality
- 22. Feasibility Studies (proof-of-concept) : • Conducted to ensure compatibility of the product or test sample with the Rapid Microbial Method (RMM) system. • May be performed in-house (using rented or loaned equipment) or at the supplier’s facility. - Avoid false positives: Samples without viable microorganisms should not yield positive results. - Avoid false negatives: Test systems must detect and quantify microorganisms as expected. - Sample matrices causing interference or incorrect results must be investigated before purchasing or validating the system. Supplier Assessment: • Suppliers should be audited similarly to raw material or equipment providers. User Requirement Specification (URS): The URS outlines system purpose, performance criteria, hardware/software needs, safety, compatibility, data management, training, supplier requirements, and budget limits. It serves as the basis for validation and ensures the system aligns with company and regulatory needs.
- 23. Validation Master Plan (VMP): • Outlines activities required to validate the RMM system for its intended use. • Includes deliverables, responsibilities, review and approval processes, and documentation to meet validation expectations. Functional Design Specification (FDS): • A detailed document outlining the functions and requirements of the RMM system. • Serves as a “validation roadmap” to ensure the system meets the user’s requirements as defined in the User Requirement Specification (URS). • Covers testing parameters necessary to validate system performance against the URS.
- 24. The FDS should also point to specific test scripts where each requirement will be evaluated and verified against pre-established acceptance criteria (e.g. what will be performed during the IQ, OQ, and PQ phases of the validation plan). • Installation Qualifcation (IQ) ensures the equipment is received as designed, safely installed, and operates in the appropriate environment. It can be performed by the RMM supplier or the end user. • Operational Qualifcation (OQ) verifies the equipment performs as intended within operational ranges, and it is the primary focus for computer system validation (CSV), including hardware, software, and security testing. • Performance Qualifcation (PQ) provides evidence that the equipment performs consistently according to predefined criteria, ensuring correct results. It includes validating the method, its suitability for the product, and demonstrating equivalence to existing methods.
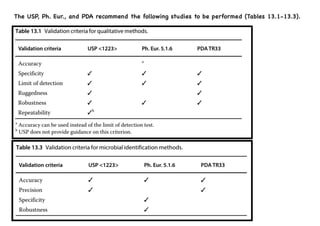
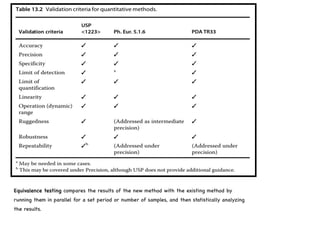
- 25. The USP, Ph. Eur., and PDA recommend the following studies to be performed (Tables 13.1-13.3).
- 26. Equivalence testing compares the results of the new method with the existing method by running them in parallel for a set period or number of samples, and then statistically analyzing the results.
- 27. References 1- Miller, M.J., 2019. Rapid microbiological methods. Pharmaceutical Microbiological Quality Assurance and Control: Practical Guide for Non-Sterile Manufacturing, pp.429-458. 2- Green, S. and Randell, C., 2016. Rapid microbiological methods explained. In Microbiological Contamination Control in Pharmaceutical Clean Rooms (pp. 169-192). CRC Press. 3- Moldenhauer, J., 2021. Rapid Methods for Pharmaceutical Processing and Their Validation. In Handbook of Validation in Pharmaceutical Processes (pp. 929-942). CRC Press.