Recombinant dna technology for food uses
- 1. Recombinant enzymes for food processing Uzair Shah Hashmi
- 2. Food-processing enzymes from recombinant microorganisms •food processing and in the production of food ingredients Enzymes traditionally isolated from culturable microorganisms, plants, and mammalian tissues are often not well-adapted to the conditions used in modern food production methods recombinant DNA technology steps in manufacture novel enzymes suitable for specific food-processing conditions. How ? • by screening microorganisms sampled from diverse environments •by modification of known enzymes using modern methods of protein engineering Advantages Improvement of microbial production strains • increase enzyme yield by deleting native genes encoding extracellular proteases • fungal production strains have been modified to reduce or eliminate their potential for production of toxic secondary metabolites
- 3. Improved pectinase production in Penicillium griseoroseum recombinant strains. Experiments have done to obtain a recombinant organism that will be having the ability to obtain pectin lyase (PL) and polygalacturonase (PG) and for that penicillium griseoroseum that produced both PL &PG simultaneously. •Firstly a strain that was reported to produce high concentration of PL was taken. •It was then transformed using pAN52pgg2 plasmid which was having a foreign gene of PG of P. grieoroseum and it was having a promoter from Aspergillus nidulans •The newly transformed P. grieoroseum T20 when checked was producing higher concentrations of both PG and PL, around 143 folds higher PL, and 15 folds greater PG. •This recombinant strain uses carbon sources of low costs that is very economical •The enzyme preparation commercially available is free of cellulolytic and proteolytic activities. •This is an efficient system that uses P. griseoroseum to express and secrete proteins.
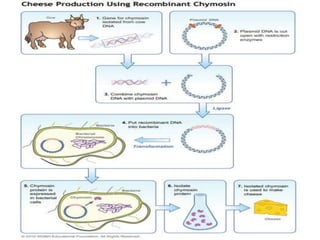
- 4. Chymosin
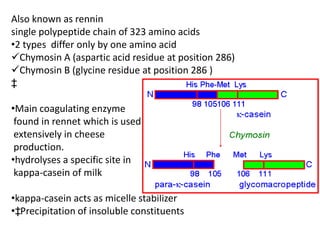
- 5. Also known as rennin single polypeptide chain of 323 amino acids •2 types differ only by one amino acid Chymosin A (aspartic acid residue at position 286) Chymosin B (glycine residue at position 286 ) ‡ •Main coagulating enzyme found in rennet which is used extensively in cheese production. •hydrolyses a specific site in kappa-casein of milk •kappa-casein acts as micelle stabilizer •‡Precipitation of insoluble constituents
- 7. Search for an alternative rennet •increasing demand •shortage of calf stomachs •ethical issues with animal slaughtering Kluyveromyces lactis increase Chymosin production has been made through expression of calf Chymosin gene in recombinant K. lactis Advantages of K. Lactis •Non toxicogenic GRAS microorganism approved by US FDA •‡Unlike p. Pastoris it does not require methanol to induce protein secretion •‡Unlike E. coli it doesn't secrete the expressed protein enclosed in inclusion bodies
- 9. comparative study of 4 different recombinant chymosins •Recombinant bovine Chymosin is the most frequently used Chymosin in the industry •new sources of recombinant Chymosin, such as goat, camel, or buffalo, are now available. When compared with other 3 enzymes •Recombinant goat Chymosin exhibited the best catalytic efficiency •recombinant goat Chymosin exhibited the best specific proteolytic activity, •recombinant goat Chymosin exhibited a wider pH range of action, •recombinant goat Chymosin exhibited a lower glycosylation degree
Editor's Notes
- #3: http://www.ncbi.nlm.nih.gov/pubmed/16769167
- #6: the chymosin content of the rennin causes a specific and rapid cleaveage of the kappa-casein component of the casein micelles. This protein stabilises the micelles and after cleavage, the casein proteins precipitate under the influence of the calcium ions. Chymosin specifically recognises the sequence from His 98 to Lys 111 and cleaves the peptide bond between Phe 105 and Met 106 in the kappa-casein chain:
- #9: http://www.inchem.org/documents/jecfa/jecmono/v28je08.htm
- #10: http://www.ncbi.nlm.nih.gov/pubmed/22281325Current strain improvement strategies have already contributed to creating more efficient and safer enzyme production strains. This trend will undoubtedly continue as the knowledge about the genetic make-up of microorganisms used for enzyme production expands and new genetic techniques emerge.