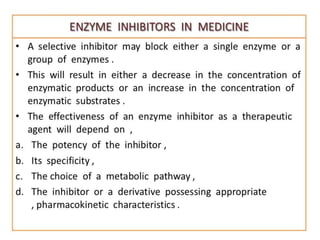
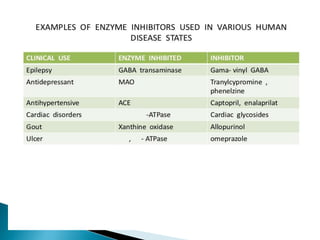
Enzyme Inhibitors
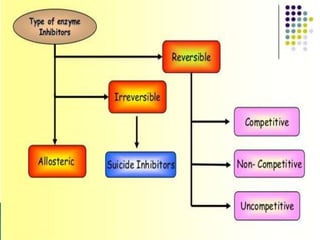
- 1. REVERSIBLE ENZYME INHIBITORS Prepared by MAHENDRA.G.S 1 M pharm Department of Pharmaceutical chemistry J S S College of Pharmacy Mysuru
- 2. ï Introduction ï Importance of enzyme inhibition ï Types of enzyme inhibitors ï Reference 2 CONTENTS
- 3. Introduction âĒ Enzyme is a protein molecule acting as catalyst in enzyme reaction. âĒ Enzyme inhibition is a science of enzyme-substrate reaction influenced by the presence of any organic chemical or inorganic metal or biosynthetic compound due to their covalent or non-covalent interactions with enzyme active site. âĒ It is well known that all these inhibitors follow same rule to interplay in enzyme reaction.
- 4. What are enzyme inhibitors? ï§ The enzyme inhibitors are low molecular weight chemical compounds. ï§ They can reduce or completely inhibit the enzyme catalytic activity either reversibly or permanently (irreversibly). ï§ Inhibitor can modify one amino acid, or several side chain(s) required in enzyme catalytic activity. ï§ In drug discovery, several drug analogues are chosen or designed to inhibit specific enzymes.
- 5. Importance of enzyme inhibition âĒ To understanding the regulation of enzyme activity within the living cells. âĒ To elucidate the kinetic mechanism of an enzyme catalyzing in a multi substrate reaction. âĒIdentification of the catalytic groups at the active site. âĒ Provide information about substrate specificity of the enzyme.
- 6. Michaelis-menten equation The michaelis-menten equation arises from the general equation for an enzymatic reaction. E+S ES E+P The michaelis menten equation is: VËģ= Where= VËģ= velocity of the reaction Vmax= maximal rate of the reaction [substrate]= conc. Of the substrate Km= michaelis-menten constant Vmax [S] km+[S] 6
- 8. ïž Inhibitor binds to Enzyme reversibly through non covalent interactions. ïž An Equilibrium is established between the free inhibitor & EI Complex and is defined by an equilibrium constant (Ki). ïž The activity of Enzyme Is fully restored on removing the Inhibitor by dialysis. 1) Reversible inhibitor:
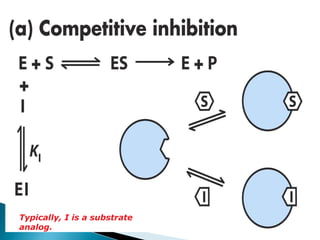
- 9. Competitive Inhibitors âĒ A competitive inhibitor often has structural features similar to the substrates whose reactions they inhibit. âĒ A I and S are in direct competition for the same binding site on the enzyme. âĒ The enzyme-bound inhibitor may either lack an appropriate functional group for further reaction/signalling or may be bound in the wrong position with respect to the catalytic residues. âĒ In any event, the enzyme- inhibitor complex (EI) is unreactive / dead-end complex.
- 10. Typically, I is a substrate analog.
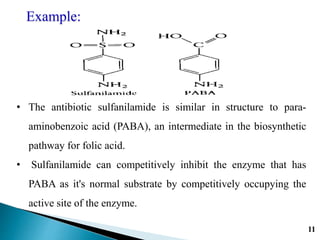
- 11. 11 Example: âĒ The antibiotic sulfanilamide is similar in structure to para- aminobenzoic acid (PABA), an intermediate in the biosynthetic pathway for folic acid. âĒ Sulfanilamide can competitively inhibit the enzyme that has PABA as it's normal substrate by competitively occupying the active site of the enzyme.
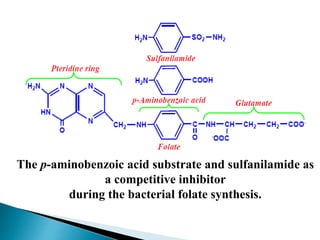
- 12. The p-aminobenzoic acid substrate and sulfanilamide as a competitive inhibitor during the bacterial folate synthesis.
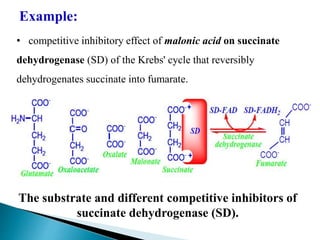
- 13. Example: âĒ competitive inhibitory effect of malonic acid on succinate dehydrogenase (SD) of the Krebs' cycle that reversibly dehydrogenates succinate into fumarate. The substrate and different competitive inhibitors of succinate dehydrogenase (SD).
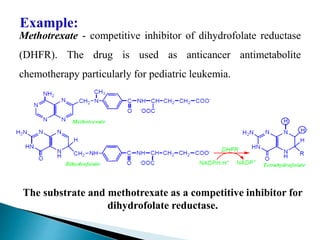
- 14. Methotrexate - competitive inhibitor of dihydrofolate reductase (DHFR). The drug is used as anticancer antimetabolite chemotherapy particularly for pediatric leukemia. Example: The substrate and methotrexate as a competitive inhibitor for dihydrofolate reductase.
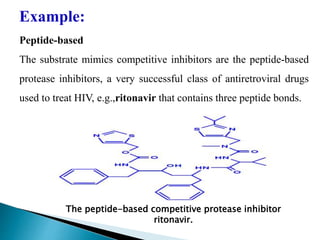
- 15. Peptide-based The substrate mimics competitive inhibitors are the peptide-based protease inhibitors, a very successful class of antiretroviral drugs used to treat HIV, e.g.,ritonavir that contains three peptide bonds. Example: The peptide-based competitive protease inhibitor ritonavir.
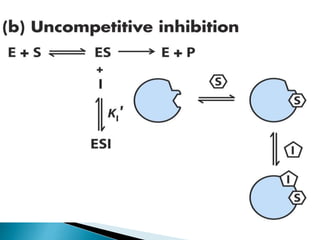
- 16. Uncompetitive Inhibitors ï― Uncompetitive inhibitors do not bind to the free enzyme. They bind only to the enzyme-substrate complex to yield an inactive ESI complex. ï― Uncompetitive inhibition is rarely observed in single- substrate reactions but is frequently observed in multisubstrate reactions. ï― An uncompetitive inhibitor can provide information about the order of binding of the different substrates. E S E.S E + P I E.S.I
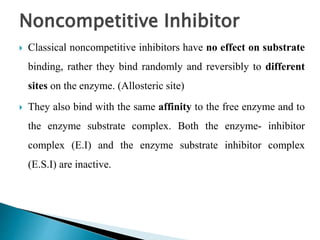
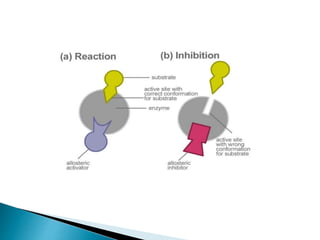
- 18. Noncompetitive Inhibitor ï― Classical noncompetitive inhibitors have no effect on substrate binding, rather they bind randomly and reversibly to different sites on the enzyme. (Allosteric site) ï― They also bind with the same affinity to the free enzyme and to the enzyme substrate complex. Both the enzyme- inhibitor complex (E.I) and the enzyme substrate inhibitor complex (E.S.I) are inactive.
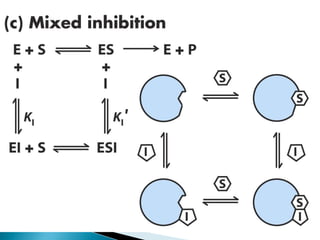
- 19. ï― These inhibitors do not affect substrate binding. ï― Again, this type of inhibition is rarely seen in single-substrate reactions. The affinity of the noncompetitive inhibitor for the free enzyme, and the enzyme-substrate complex, are different. ï― These non-ideally behaving noncompetitive inhibitors are called mixed-type inhibitors.
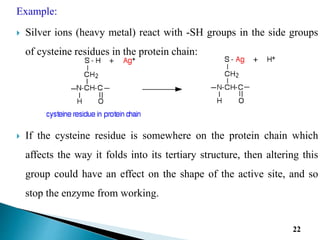
- 22. Example: ï― Silver ions (heavy metal) react with -SH groups in the side groups of cysteine residues in the protein chain: ï― If the cysteine residue is somewhere on the protein chain which affects the way it folds into its tertiary structure, then altering this group could have an effect on the shape of the active site, and so stop the enzyme from working. 22
- 25. References âĒAshutosh Kar. Medicinal Chemistry (Fifth revised & Expandded edition), New Age International Publishers. 2010: 917-972 âĒBurgerâs. Medicinal Chemistry & Drug Discovery. Sixth Edition (Volume 1). A john wiley and sond, Inc., Publiction, new jersey. 2003: 715-774 âĒAlbert L. Lehninger. Biochemistry (Second edition), Worth Publishers, Inc. New York. 1979: 189-195


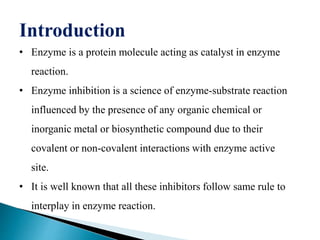


![Michaelis-menten equation
The michaelis-menten equation arises from the
general equation for an enzymatic reaction.
E+S ES E+P
The michaelis menten equation is:
VËģ=
Where=
VËģ= velocity of the reaction
Vmax= maximal rate of the reaction
[substrate]= conc. Of the substrate
Km= michaelis-menten constant
Vmax [S]
km+[S]
6](https://image.slidesharecdn.com/evalvationsaminar-171121162144/85/Enzyme-Inhibitors-6-320.jpg)