Revive Brochure
- 1. Adda Plot, Sharaiz Avenue, Jati Umra Road, Off Raiwind Road, Lahore. P: +92 42 353 22612-6 F: +92 42 353 22618 E: info@revivepharma.com W: www.revivepharmakon.com
- 2. Revive Pharmakon is a family-owned business that provides a diverse range of IV Solutions for maintaining the electrolyte balance and supplementing carbohydrate levels in the human body. Revive focuses on producing superior quality products to serve the needs of the global pharmaceutical IV industry. Revive Pharmakon was established for the primary purpose of providing the highest quality healthcare solutions, to those most in need. Today, the quality of healthcare drugs (IV solutions) that are prevalent in the developing regions barely meet the world quality standards required, leading to a shortage in supply of trustworthy and effective products. Hence, Revive was erected on the ideology and vision to provide these regions with healthcare products of the highest quality in compliance with global health standards. In order to be able to manufacture these world standard products, Reviveâs manufacturing facility, located in Lahore, Pakistan, is equipped with state-of-the art equipment that ensure extremely reliable, safe and efficient processes. Moreover, it is managed by exceptionally skilled and qualified professionals that strive to produce high quality products using innovative and advanced technologies. Revive believes in systems and efficient manufacturing practices. It is an ISO:9001 certified company. To make itâs process more efficient it has put in place the SAP / ERP platform that ensures smooth day-to-day processes and procedures and capturing of real-time information for executive decision-making. Moreover, the PLC based computerized environment with data printing arrangements brings us one step further to ensuring quality. Revive prides itself in being an environmentally conscious and socially responsible organization, and is certified under ISO:14001. Furthermore, in itâs resolute commitment to provide a safe and healthy work environment for all itâs employees, Revive has complied with and obtained the OHSAS:18001 certification. The Research & Development Unit at Revive Pharmakon, is dedicated to continuously evolving and updating its processes and product offerings in accordance with the dynamic and ever changing Global Pharmaceutical Industry. In the near future, this R&D unit plans on expanding the organizationâs product profile into Antibiotics, Dialysis and Vaccines. COMPANY BACKGROUND
- 3. OUR VISION OUR QUALITY POLICY Revive Pharmakon is committed to becoming a leading, reputable and trusted corporation in the Global Pharmaceutical Industry through its efforts in improving access and quality of healthcare, by providing safe, effective and reliable products, manufactured by extremely skilled and qualified professionals. Through our continuous investments in technology, human resources, systems and processes, we strive to achieve and ensure phenomenal levels of quality, service, efficiency, safety and environmental consciousness. At Revive Pharmakon, we take pride in providing our customers with world-class quality products that are safe and compliant with all required regulatory parameters. We ensure that the focus of our organization is always on achieving the highest-quality product standards for our diverse and global target markets. We give the utmost importance to the safety and quality of products manufactured and therefore elaborate quality checks comprising physical, chemical and microbiological tests are conducted at all stages of manufacturing. Our Quality Control lab has successfully conducted the stability studies on trial batches of pilot scale, for our various products. Furthermore, the trial batches have been successfully tested in our internal lab as well as other external accredited labs.
- 4. PRODUCTION FACILITY Sr. # 1 2 3 4 5 6 7 8 9 SOLUTION PREPARATION TANKS BOTTLE PACK (ROMMELAG) LEAK TEST MACHINE (WILCOMAT) EURO CAP MACHINES AUTOCLAVES WATER SOFTENER, RO PLANT & DISTILLATION UNIT OPTICAL CHECKING MACHINES LABELLING MACHINES PILLOW PACK MACHINES MACHINERY Reviveâs manufacturing facility & lab located on Jatti Umra Road in Lahore, Pakistan, is furnished with state-of the art equipment. Some of the machinery includes: Furthermore, the facility is also well-equipped with multiple and separate storage facilities each for its raw materials, packaging, finished goods and finished good quarantine requirements with holding capacity of about 2.7 Million bottles. It also has separate storage areas of any rejected or recalled products. Reviveâs manufacturing facility is also equipped with the following utilities: Self-sufficient in Water Resources Multiple Effect Distillation Unit with a capacity of 3500 Liters per hour 12,000 Liters Water for Injection Storage Tank with a loop system Pharmaceutical industry accepted Air handling Units (AHUs) to maintain the HVAC/R flow 69 HEPA Filters More that 20 âAir Changesâ per hour of all the areas Class 100 facility with SS walls and ceiling in SP & BP rooms Air Curtains on all entry points to avoid contamination HPLC, TOC, UV/Visible Spectrophotometer, Flame Photometer, Digital Polarimeter, FTIR, Particle Counter, Stability Chamber, Conductivity Meter, Colony Counter, PH Meter, Filteration Assemblies.
- 5. *All products are available with or without Eurocaps. Revasol 5% 100ml Dextrose 5% Blood substitute, electrolytes, haematinics, carbohydrate and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 100 ml 1. Revasol 5% 500ml Dextrose 5% Blood substitute, electrolytes, haematinics, carbohydrate and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 2. REVASOL 5% Revasol 5% 1000ml Dextrose 5% Blood substitute, electrolytes, haematinics, carbohydrate and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 3.
- 6. *All products are available with or without Eurocaps. Revasol 10% 100ml Dextrose 10% Blood substitute, electrolytes, haematinics and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 1. Revasol 10% 500ml Dextrose 10% Blood substitute, electrolytes, haematinics and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 2. REVASOL 10% Revasol 10% 1000ml Dextrose 10% Blood substitute, electrolytes, haematinics and water replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 3.
- 7. *All products are available with or without Eurocaps. REVASOL HD 1. Revasol HD 100mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg, Dextrose anhydrous 5gm/100ml Isotonic volume expander, Electrolyte replacement. Lactated Ringerâs and 5% Dextrose Injection, has value as a source of water, electrolytes, and calories. 2. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg, Dextrose anhydrous 5gm/ 100ml Revasol HD 500ml Isotonic volume expander, Electrolyte replacement. Lactated Ringerâs and 5% Dextrose Injection, has value as a source of water, electrolytes, and calories. 3. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 1000 ml Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg, Dextrose anhydrous 5gm/ 100ml Revasol HD 1000ml Isotonic volume expander, Electrolyte replacement. Lactated Ringerâs and 5% Dextrose Injection, has value as a source of water, electrolytes, and calories. 100 ml 500 ml
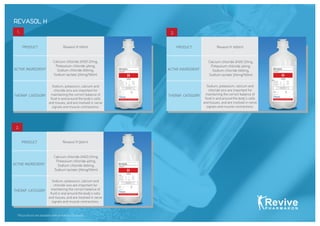
- 8. *All products are available with or without Eurocaps. REVASOL H 2. Revasol H 500mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Sodium, potassium, calcium and chloride ions are important for maintaining the correct balance of fluid in and around the bodyâs cells and tissues, and are involved in nerve signals and muscle contractions. Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg/100ml 3. Revasol H 1000mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Sodium, potassium, calcium and chloride ions are important for maintaining the correct balance of fluid in and around the bodyâs cells and tissues, and are involved in nerve signals and muscle contractions. Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg/100ml 1. Revasol H 100mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Calcium chloride 2H2O 27mg, Potassium chloride 40mg, Sodium chloride 600mg, Sodium lactate 310mg/100ml Sodium, potassium, calcium and chloride ions are important for maintaining the correct balance of fluid in and around the bodyâs cells and tissues, and are involved in nerve signals and muscle contractions. 100 ml 500 ml 1000 ml
- 9. *All products are available with or without Eurocaps. 3. Revasol NS 1000mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Sodium chloride 0.9% Isotonic volume expander, Electrolyte replacement. Revasol NS 100mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Sodium chloride 0.9% Isotonic volume expander, Electrolyte replacement. 1. 100 ml 1000 ml 2. Revasol NS 500mlPRODUCT ACTIVE INGREDIENT THERAP. CATEGORY Sodium chloride 0.9% Isotonic volume expander, Electrolyte replacement. 500 ml REVASOL NS
- 10. REVASOL DN 2. Revasol DN 1000ml Sodium chloride 0.9% + dextrose 5% Isotonic volume expander, Electrolyte replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 1. Revasol DN 100ml Sodium chloride 0.9% + dextrose 5% Isotonic volume expander, Electrolyte replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 3. Revasol DN 500ml Sodium chloride 0.9% + dextrose 5% Isotonic volume expander, Electrolyte replacement. PRODUCT ACTIVE INGREDIENT THERAP. CATEGORY 100 ml 500 ml 1000 ml *All products are available with or without Eurocaps.