Risk analysis of a compounding pharmacy
- 1. Microbiological Risk Analysis And Compounding Pharmacies Anthony Grilli MS FOCUS Scientific November 14, 2013 Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 2. New England Compounding Center This time last year ŌĆ” an adulterated drug, contaminated with a common mold was administered to 1000ŌĆÖs of patients across the country, killing 64 people and sickening more than 750 with persistent fungal infections. And the impact continues to unfold. How did this happen? Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 3. Perfect Storm! Drug Company Mold Bring your project into FOCUS for a clear resolution Regulator www.focus-sci.com User
- 4. Debate Over Regulations ’üĮ ’üĮ ’üĮ ’üĮ ’üĮ Compounding pharmacies are FDA Registered, but not FDA regulated They are inspected by State Departments of Health Not cGMP USP <797> Pharmaceutical Compounding ŌĆō Sterile Preparations Federal oversight may be coming: Sterile products produced in advance of or without a prescription and shipped interstate should be subject to the highest level of controls, established by FDA and appropriate to the activity, similar to cGMP standards applicable to conventional drug manufacturers.ŌĆØ ’é© Statement of Margaret A. Hamburg, M.D., Commissioner of Food and Drugs, April 16, 2013. ’üĮ" Bring your project into FOCUS for a clear resolution
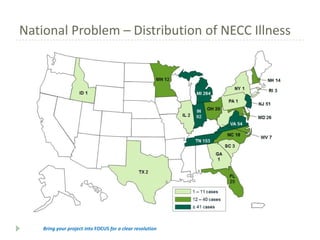
- 5. National Problem ŌĆō Distribution of NECC Illness Bring your project into FOCUS for a clear resolution
- 6. Compounding Centers Provide Benefits to Patients ’üĮ Allows medication to be personalized for an individual patient ’üĮ ’üĮ ’üĮ Can make medications more palatable ’üĮ ’üĮ Liquid or topical form for patients who canŌĆÖt swallow pills Can formulate medications that large pharma manufacturers have discontinued ’üĮ ’üĮ Take out unpleasant flavors ŌĆō important for children, elderly, pets Can make medications in formulations that may not be available from mass manufacturers ’üĮ ’üĮ Can remove allergens from product (lactose, dyes, preservatives, glutens) Future of medicine will be personalized doses Product may still be needed by thousands of patients, but not profitable to make on large scale any longer. Can bypass the FDAŌĆÖs long approval and inspection process, for patients that have an immediate unique need. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 7. Other Compounding Problems ’üĮ Product Recalls and contamination ’üĮ Med Prep Consulting ’üĮ ’üĮ Main Street Compounding, TN ’üĮ ’üĮ Nationwide recall ŌĆō 5 patients with eye infections Compounding Shop, FL ’üĮ ’üĮ Nationwide recall of products FDA found product contaminated with fungus and bacteria Clinical Specialties, GA ’üĮ ’üĮ Nationwide recall of all sterile products after hospital discovered mold in unopened IV solution. Recall Budensonide, not sterile Specialty Compounding, TX ’üĮ Rhodococcus equi Bring your project into FOCUS for a clear resolution
- 8. How big is the problem? ’üĮ ’üĮ 53,000 compounding pharmacies in the US According to recent estimates, there are about 3,000 compounding pharmacies practicing sterile compounding in the US : (National Conference of State Legislatures June 2013 Kara Hinkley) ’üĮ ’üĮ ’üĮ ’üĮ FDA started auditing these compounding centers last year. To date, almost 60 US FDA 483 responses posted: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryO perationsandPolicy/ORA/ORAElectronicReadingRoom/ucm340853.htm The response back to the FDA to these 483ŌĆÖs has been predominantly ŌĆō we donŌĆÖt have to follow GMPŌĆÖs and you have no authority over us. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 9. LetŌĆÖs do a thought experiment ŌĆ”.. Mega Compounding Corp manufactures sterile methylprednisilone for intrathecal injection to treat back pain. Components are received as non-sterile ingredients, they are compounded, sterilized and filled to vials with elastomeric closures. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 10. Reducing Microbial Risk of Contaminated Product Hurdles to Microbial Contamination and Illness Formulation Water Activity Kill steps Bring your project into FOCUS for a clear resolution pH Preservatives www.focus-sci.com
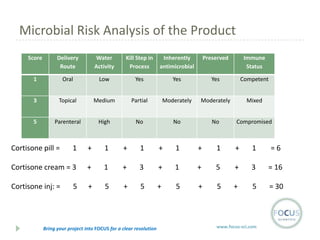
- 11. Microbial Risk Analysis of the Product Score Delivery Route Water Activity Kill Step in Process Inherently antimicrobial Preserved Immune Status 1 Oral Low Yes Yes Yes Competent 3 Topical Medium Partial Moderately Moderately Mixed 5 Parenteral High No No No Compromised Cortisone pill = 1 + 1 + 1 + 1 + 1 + 1 =6 Cortisone cream = 3 + 1 + 3 + 1 + 5 + 3 = 16 Cortisone inj: = + 5 + 5 + 5 + 5 + 5 = 30 5 Bring your project into FOCUS for a clear resolution www.focus-sci.com
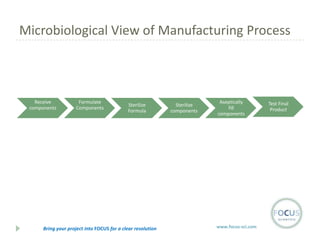
- 12. Microbiological View of Manufacturing Process Receive components Formulate Components Sterilize Formula Bring your project into FOCUS for a clear resolution Sterilize components Aseptically fill components www.focus-sci.com Test Final Product
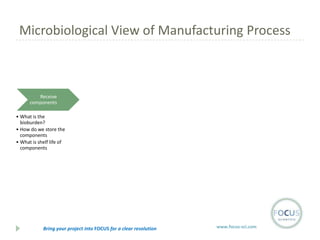
- 13. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó How do we store the components ŌĆó What is shelf life of components Bring your project into FOCUS for a clear resolution www.focus-sci.com
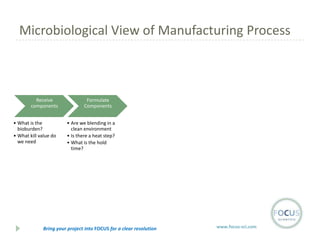
- 14. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó What kill value do we need Formulate Components ŌĆó Are we blending in a clean environment ŌĆó Is there a heat step? ŌĆó What is the hold time? Bring your project into FOCUS for a clear resolution www.focus-sci.com
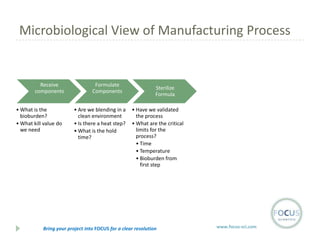
- 15. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó What kill value do we need Formulate Components ŌĆó Are we blending in a clean environment ŌĆó Is there a heat step? ŌĆó What is the hold time? Sterilize Formula ŌĆó Have we validated the process ŌĆó What are the critical limits for the process? ŌĆó Time ŌĆó Temperature ŌĆó Bioburden from first step Bring your project into FOCUS for a clear resolution www.focus-sci.com
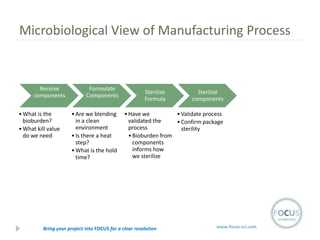
- 16. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó What kill value do we need Formulate Components ŌĆó Are we blending in a clean environment ŌĆó Is there a heat step? ŌĆó What is the hold time? Sterilize Formula Sterilize components ŌĆó Have we ŌĆó Validate process validated the ŌĆó Confirm package process sterility ŌĆó Bioburden from components informs how we sterilize Bring your project into FOCUS for a clear resolution www.focus-sci.com
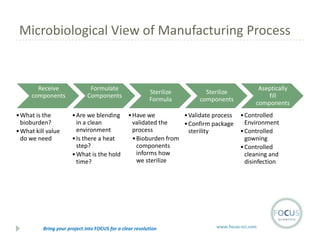
- 17. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó What kill value do we need Formulate Components ŌĆó Are we blending in a clean environment ŌĆó Is there a heat step? ŌĆó What is the hold time? Sterilize Formula Sterilize components ŌĆó Have we ŌĆó Validate process validated the ŌĆó Confirm package process sterility ŌĆó Bioburden from components informs how we sterilize Bring your project into FOCUS for a clear resolution Aseptically fill components ŌĆó Controlled Environment ŌĆó Controlled gowning ŌĆó Controlled cleaning and disinfection www.focus-sci.com
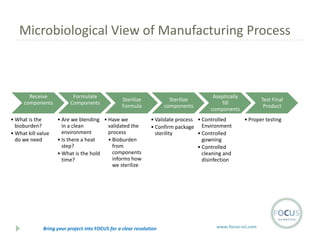
- 18. Microbiological View of Manufacturing Process Receive components ŌĆó What is the bioburden? ŌĆó What kill value do we need Formulate Components Sterilize Formula ŌĆó Are we blending ŌĆó Have we in a clean validated the environment process ŌĆó Is there a heat ŌĆó Bioburden step? from components ŌĆó What is the hold informs how time? we sterilize Sterilize components Aseptically fill components ŌĆó Validate process ŌĆó Controlled ŌĆó Confirm package Environment sterility ŌĆó Controlled gowning ŌĆó Controlled cleaning and disinfection Bring your project into FOCUS for a clear resolution Test Final Product ŌĆó Proper testing www.focus-sci.com
- 19. Environmental Monitoring Risk Analysis: ’üĮ Microflora changes with seasons ’üĮ ’üĮ ’üĮ HVAC is in constant state of flux ’üĮ ’üĮ ’üĮ More mold in fall More bacteria from skin in dryer months Filters fail or become saturated Room pressures changes with doors opening and closing People and materials change over time Control Point: ’üĮ Perform EM every day you manufacture USP <797>: ’üĮ Perform EM twice a year Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 20. Gowning ’üĮ Risk Analysis: ’üĮ ’üĮ ’üĮ ’üĮ ’üĮ ’üĮ Control point ’üĮ ’üĮ People are the greatest contributor to contamination Each person brings 100,000,000,000,000 germs in the clean room with them. People shed 100,000 particles per minute A simple nod contributes 50,000 particles Technicians must lean into hoods to fill product Full sterile gown with no skin or street clothes showing. USP <797> ’üĮ Lab coat, gloves, hair net. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 21. Gowning Validation ’üĮ ’üĮ Microbial samples of gowns are taken to verify outside of gowns and gloves are still clean USP <797> ’üĮ ’üĮ ’üĮ ’üĮ Only gloves are sampled Only sampled immediately after garbing Only sampling gloves Microbiologists know: ’üĮ ’üĮ ’üĮ Other body parts become contaminated and can contaminate inner core Glove contamination occurs DURING manufacturing (touching head, etc) Should be run as a check every day Bring your project into FOCUS for a clear resolution www.focus-sci.com
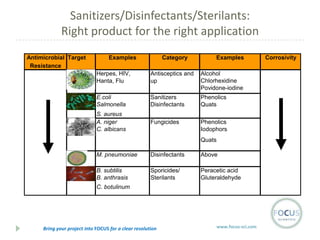
- 22. Sanitizers/Disinfectants/Sterilants: Right product for the right application Antimicrobial Target Resistance Organis Examples Category Examples Herpes, HIV, Hanta, Flu Antisceptics and up Alcohol Chlorhexidine Povidone-iodine E.coli Salmonella Sanitizers Disinfectants Phenolics Quats S. aureus A. niger C. albicans Fungicides Phenolics Iodophors Quats M. pneumoniae Disinfectants Above B. subtilis B. anthrasis Sporicides/ Sterilants Peracetic acid Gluteraldehyde C. botulinum Bring your project into FOCUS for a clear resolution www.focus-sci.com Corrosivity
- 23. Disinfection ’üĮ Risk Analysis ’üĮ ’üĮ ’üĮ ’üĮ ’üĮ ’üĮ Control Point: ’üĮ ’üĮ Sterile processing means no organisms Spore forming bacillus common air contaminant Mold spores common air contaminant Manufacturing surfaces differ in their ability to protect microbes from disinfection. Microbes differ in their ability to resist disinfection. Validate sporicide efficacy on manufacturing materials using environmental isolates. USP <797>: ’üĮ ’üĮ Not specific about sporicide usage No mention of validation Bring your project into FOCUS for a clear resolution
- 24. Finished Product Testing ’üĮ ’üĮ Even more critical in an environment of loose control. USP <797> does not require Sterility Testing on all aseptically produced products that ’üĮ ’üĮ ’üĮ Only required on products that are ŌĆ£high riskŌĆØ which means they were not sterile on receipt or experienced a processing deviation. Not needed on products that are ŌĆ£aseptically processedŌĆØ in a controlled environment and are stored for 48 hours at CRT. USP <797> allows for release prior to test results. Bring your project into FOCUS for a clear resolution
- 25. What happened at Mega Compounding Corp? ’üĮ ’üĮ ŌĆ£They did what they were told, they followed the compendiaŌĆØ But unfortunately, ’üĮ ’üĮ ’üĮ ’üĮ Inadequate gowning Incomplete monitoring of environmental microbes Inadequate disinfection Insufficient testing Bring your project into FOCUS for a clear resolution
- 26. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 27. Conclusion ’üĮ ’üĮ ’üĮ cGMP or not ŌĆō hazard analysis, critical control point identification, and critical limit setting will ensure a safe product. No regulation can cover every situation. The compounding pharmacy debacle shows: ’üĮ ’üĮ Importance of cGMP to ensuring drug quality Importance of applying sound quality and scientific evaluation to ensure drug quality. Bring your project into FOCUS for a clear resolution www.focus-sci.com
- 28. Questions? Anthony Grilli MS Principal Consultant FOCUS Scientific Services LLC agrilli@focus-sci.com (973)216 6039 Bring your project into FOCUS for a clear resolution www.focus-sci.com