Sampath
- 1. Development of RP-HPLC method for Simultaneous estimation of Bendamustine Hydrochrolide and Omeprazole in Injection formulation Under the esteemed guidance of Sri B. Thangabalan M.pharm, (Ph.d) Presented by K. Sampath Kumar Y11MPH18060 SIMS COLLEGE OF PHARMACY 1 GUNTUR.
- 2. Aim & Objective The literature survey indicates that there are no methods reported for the simultaneous estimation of Bendamustine Hydrochrolide and Omeprazole by RP – HPLC. The present work is undertaken with an objective to develop & validate a simple, sensitive, economical , precise & accurate chromatographic method by HPLC.
- 3. Bendamustine Hydrochloride: Description: white microcrystalline powder Mol. Formula:C16H21Cl2N3O2 Mol. Weight: 358.262gm/mol Iupac Name: (4-{5-[bis-(2-chloroethyl) amino]-1-methyl- 1Hbenzimidazol-2-yl} butanoic acid) Cl-H2C-H2C N N Cl-H2C-H2C . (CH2)3-COOH HCl N CH3
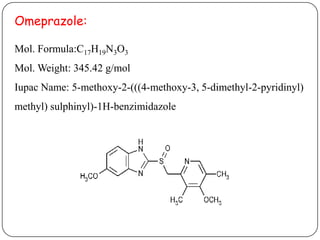
- 4. Omeprazole: Mol. Formula:C17H19N3O3 Mol. Weight: 345.42 g/mol Iupac Name: 5-methoxy-2-(((4-methoxy-3, 5-dimethyl-2-pyridinyl) methyl) sulphinyl)-1H-benzimidazole
- 5. UV method: 1. M. Mathrusri Annapurna et.al Derivative Spectrophotometric Methods for the Determination of Bendamustine Hydrochloride 2. M. Mathrusri Annapurna et.al New analytical methods for the determination of Bendamustine hydrochloride: An anti-neoplastic drug 3. D.Kumaraswamy Statistical assurance of process validation by analytical method development and validation for omeprazole capsules and blend. 4. Satish d. Bhuva, mehul m. Patel spectrophotometric simultaneous estimation of omeprazole and cinitapride in bulk and formulation 5. Nayan M Jagani et.al Development and Validation of Dual Wavelength Method For Simultaneous Estimation of Omeprazole and Cinitapride in Combined Capsule Dosage Form. 6. Y.G. Makani et.al development and validation of first order derivative Spectrophotometric method for simultaneous estimation of Omeprazole and cinitapride in pharmaceutical dosage form
- 6. HPLC: 7. Mathrusri Annapurna M. et.al Stability-Indicating Liquid Chromatographic Method for the Determination of Bendamustine Hydrochloride in Parenterals 8. E. Sasi Kiran Goud et. al Development and validation of RP-HPLC method for determination of related substances of bendamustine hydrochloride in bulk drug 9. Kalakonda Sri Nataraj et.al Development and validation of RP-HPLC method for the estimation of omeprazole in bulk and capsule dosage forms 10. S. S. Pujeri et.al Development and Validation of LC Method for the Assay of Omeprazole Enantiomers in Pharmaceutical Formulations
- 7. Plan of Work 1. Literature Survey 2. Selection of multi-component formulation 3. Procurement of selected drug for study 4. Selection of mobile phase 5. Optimization of chromatographic conditions A. selection of column B. selection of column temperature C. selection of flow rate D. fixing of injection volume E. fixing of detection wave length 6. Validation i. Accuracy iv. Linearity ii. Precision v. LOD/LOQ iii. Specificity vi. Ruggedness vii. Robustness
- 8. References: 1. http://en.wikipedia.org/wiki/ 2. www.chem.ucla.edu~bacherUV-visuv



![Bendamustine Hydrochloride:
Description: white microcrystalline powder
Mol. Formula:C16H21Cl2N3O2
Mol. Weight: 358.262gm/mol
Iupac Name: (4-{5-[bis-(2-chloroethyl) amino]-1-methyl-
1Hbenzimidazol-2-yl} butanoic acid)
Cl-H2C-H2C
N N
Cl-H2C-H2C
.
(CH2)3-COOH HCl
N
CH3](https://image.slidesharecdn.com/sampath-130320082012-phpapp02/85/Sampath-3-320.jpg)