Section 3 .pptx
- 1. MATERIAL SCIENCE ENG. KAREEM. H. MOKHTAR
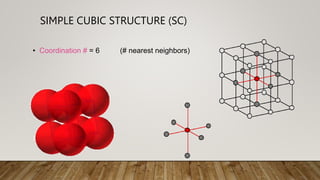
- 3. ŌĆó Coordination # = 6 (# nearest neighbors) SIMPLE CUBIC STRUCTURE (SC)
- 4. ŌĆó Coordination # = 8 ŌĆó Atoms touch each other along cube diagonals. BODY CENTERED CUBIC STRUCTURE (BCC)
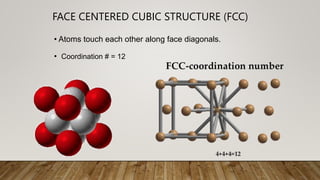
- 5. ŌĆó Coordination # = 12 ŌĆó Atoms touch each other along face diagonals. FACE CENTERED CUBIC STRUCTURE (FCC)
- 6. ATOMIC PACKING FACTOR (APF) APF = Volume of atoms in unit cell* Volume of unit cell *assume hard spheres
- 7. close-packed directions a R=0.5a contains 8 x 1/8 = 1 atom/unit cell APF = a3 4 3 p (0.5a) 3 1 atoms unit cell atom volume unit cell volume = 0.52 ATOMIC PACKING FACTOR: SCC
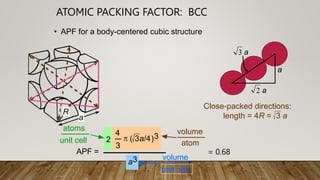
- 8. ATOMIC PACKING FACTOR: BCC a APF = 4 3 p ( 3a/4)3 2 atoms unit cell atom volume a3 unit cell volume length = 4R = Close-packed directions: 3 a ŌĆó APF for a body-centered cubic structure a R a 2 a 3 = 0.68
- 9. ATOMIC PACKING FACTOR: FCC maximum achievable APF APF = 4 3 p ( 2a/4)3 4 atoms unit cell atom volume a3 unit cell volume Close-packed directions: length = 4R = 2 a Unit cell contains: 6 x1/2 + 8 x1/8 = 4 atoms/unit cell a 2 a =0.74
- 10. ALLOYS
- 11. PHASE DIAGRAM A phase diagram shows the phases and their compositions at any combination of temperature and alloy composition
- 12. SOLUBILITY ŌĆó how much of each material or component we can combine without producing an additional phase ŌĆó Unlimited Solubility ŌĆó When we pour a substance into another and stir, and only one phase is produced regardless the quantity. ŌĆó Limited Solubility ŌĆó when we pour a substance into another and stir, and two phases is produced after a certain amount.
- 13. CONDITIONS FOR UNLIMITED SOLID SOLUBILITY ŌĆó the Hume-Rothery rules, are as follows: ŌĆó 1. Size factor: The atoms or ions must be of similar size, with no more than a 15% difference in atomic radius ŌĆó 2. Crystal structure: The materials must have the same crystal structure ŌĆó 3. Valence: The ions must have the same valence ŌĆó 4. Electronegativity: The atoms must have approximately the same electronegativity.
- 14. BINARY PHASE DIAGRAMS ŌĆó When only two elements or two compounds are present in a material, a binary phase diagram can be constructed.
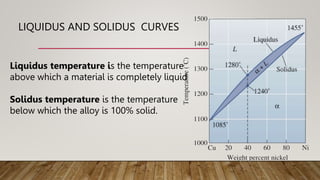
- 15. LIQUIDUS AND SOLIDUS CURVES Liquidus temperature is the temperature above which a material is completely liquid Solidus temperature is the temperature below which the alloy is 100% solid.
- 17. HOW TO DETERMINE A COMPOSITION A tie line is a horizontal line within a two-phase region drawn at the temperature of interest
- 18. QUESTION 1 Determine the composition of each phase in a Cu-40% Ni alloy at 1300┬░C, 1270┬░C, 1250┬░C, and 1200┬░C
- 19. SOLUTION 1300┬░C: Only liquid is present The liquid must contain 40%Ni 1270┬░C:Two phases are present the liquid contains 37% Ni, and the solid contains 50% Ni. 1250┬░C: two phases are present. the liquid contains 32% Ni, and the solid contains 45% Ni. 1200┬░C: Only solid is present, so the solid must contain 40% Ni.