1 of 18



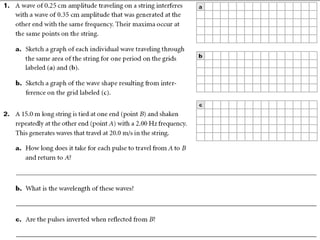
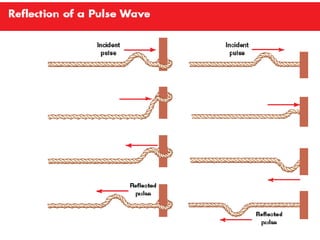
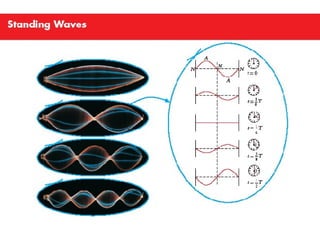
Ad
Recommended
Prova slideshare
Prova slideshareCristina Clementoni
Ã˝
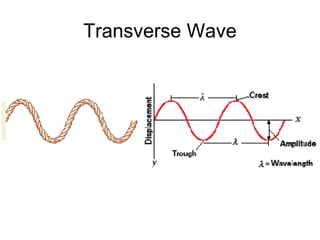
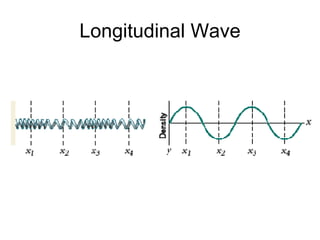
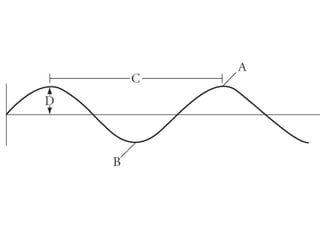
Le onde sono perturbazioni che si propagano, con onde trasversali dove lo spostamento del mezzo è perpendicolare alla direzione di propagazione e onde longitudinali dove lo spostamento avviene nella stessa direzione. Le onde nell'acqua sono una combinazione di entrambi i tipi. Il periodo è il tempo che una lunghezza d'onda impiega a passare per un punto, con la frequenza calcolata come f = 1 / t.Engr 214 notes 5 1 2013
Engr 214 notes 5 1 2013dalidala
Ã˝
The document is a lecture outline for Dynamics (lectures 5) taught by Gang Chen at Marshall University. It discusses kinetics of particles using energy and momentum methods, focusing on using conservation of energy and momentum to solve for unknowns related to particle motion.Equations of motion normal & tangential
Equations of motion normal & tangentialGrace Palermo
Ã˝
This document discusses equations of motion for a particle moving along a curved path. It explains that the equation of motion can be written in terms of normal and tangential coordinates, with forces represented in the normal, tangential, and binormal directions. The centripetal force acts in the normal direction towards the center of the path. Examples are provided to demonstrate solving equations of motion for situations involving circular motion with various constraints.Kinetics of particles
Kinetics of particlesGrace Palermo
Ã˝
This document discusses the kinetics of particles and Newton's laws of motion. It explains that the two main factors affecting an object's motion are the forces acting on it and its mass. Newton's second law states that the acceleration of an object is directly proportional to the net force acting on it and inversely proportional to its mass. The equation F=ma expresses this relationship, where F is the net force, m is the mass, and a is the acceleration. The document also provides examples demonstrating applications of Newton's laws to problems involving blocks connected by cords and pulleys.008 newton's second law of motion
008 newton's second law of motionphysics101
Ã˝
This document discusses kinetics, which is the study of how unbalanced forces affect motion. It explains Newton's three laws of motion, with a focus on Newton's second law, which relates force, mass, and acceleration. Formulas for force, weight, and the vector sum of forces acting on a particle are provided. Several example problems demonstrate how to apply these concepts to calculate accelerations, tensions, speeds, and distances given forces and masses.Engr 214 notes 5 3 2013
Engr 214 notes 5 3 2013dalidala
Ã˝
This document is from Gang Chen's lecture notes on Dynamics (lecture 5). The lecture discusses kinetics of particles and covers energy and momentum methods for analyzing the motion of particles. It focuses on using energy and momentum concepts to solve problems involving particles.Kinetics of particles work and energy
Kinetics of particles work and energyGrace Palermo
Ã˝
This document discusses work, energy, and their application to solving kinetics problems involving forces, velocities, and displacements. It defines work as the product of force and displacement. It also defines kinetic energy as the energy of motion and potential energy as energy due to position or gravitational interaction. The principle of work and energy states that the net work done on an object equals its change in kinetic energy. Examples are provided to demonstrate how to apply these concepts to calculate velocities, distances, tensions, and work in problems involving forces on moving objects.Circular and gavitational force
Circular and gavitational forceeshwar360
Ã˝
1. The document discusses Newton's laws of motion and gravity and how they can be used to derive Kepler's laws of planetary motion.
2. It shows that assuming a central force directed at a central body, the conservation of angular momentum implies that equal areas are swept out in equal times, in accordance with Kepler's second law.
3. By applying Newton's gravity equation and conservation of angular momentum to the Lagrangian of a central-force problem, the document derives that the differential equation governing planetary orbits is identical in form to that of an ellipse, explaining Kepler's first law that planets follow elliptical orbits.Chapter 13 kinetics_of_particle--force_acceleration
Chapter 13 kinetics_of_particle--force_accelerationSelf-employed
Ã˝
This document discusses kinetics of particles and Newton's laws of motion. It introduces the concept of equation of motion relating the forces acting on a particle to its acceleration. Equations of motion are developed for rectangular, normal-tangential, and cylindrical coordinate systems. Examples are provided to demonstrate solving equations of motion for particles undergoing accelerated motion under various force conditions in different coordinate systems.Tutorial 1
Tutorial 1Kumutha Danasakaran
Ã˝
The document covers various topics in mechanics including statics, dynamics, kinematics, kinetics, scalars, vectors, trigonometry functions, geometry, and Newton's laws of motion. It defines concepts like particles, rigid bodies, and non-rigid bodies. It also explains the parallelogram law for adding vectors and how to use trigonometric functions and rules to solve problems involving forces and motion.Kinetics of a Particle : Force and Acceleration
Kinetics of a Particle : Force and AccelerationAbduljalil AlAbidi
Ã˝
Here are the key steps to solve this problem:
1) Draw a free body diagram of each block, showing all external forces.
2) Write the equation of motion for each block in the x and y directions: ΣFx = max, ΣFy = may
3) The tension in each cable will be the same. Substitute this into the equations of motion.
4) Solve the equations simultaneously to find the acceleration and tension.
The acceleration and tension can be determined by setting up and solving the simultaneous equations of motion for each block based on Newton's 2nd law. Friction and the coefficient of kinetic friction must be accounted for between block C and the horizontal surface.Kinetics of particle
Kinetics of particlebahria university islamabad
Ã˝
This document contains a presentation on Newton's second law of motion. The presentation topics include the relation between force, mass and acceleration, applications of Newton's second law, equations of motion, and an introduction to kinetics of particles. The document provides definitions and explanations of key concepts such as force, mass, acceleration, momentum, impulse, and kinetics. It also includes sample problems demonstrating applications of Newton's second law and equations of motion, along with step-by-step solutions. The presentation was made by Danyal Haider and Kamran Shah and covers fundamental principles of classical mechanics.Chapter 12 kinematics_of_a_particle
Chapter 12 kinematics_of_a_particleSelf-employed
Ã˝
This document discusses the kinematics of particles in rectilinear and curvilinear motion. It defines key concepts like position, displacement, velocity, and acceleration for both continuous and erratic rectilinear motion. Examples are provided to demonstrate how to construct velocity-time and acceleration-time graphs from a given position-time graph, and vice versa. The chapter then discusses general curvilinear motion, defining position, displacement, velocity, and acceleration using vector analysis since the curved path is three-dimensional. Fundamental problems and practice problems are also included.Chapter 11 kinematics of particles
Chapter 11 kinematics of particlesRogin Beldeneza
Ã˝
This document discusses kinematics concepts related to particle dynamics, including:
1) Rectilinear and curvilinear motion, describing the position, velocity, and acceleration of particles moving along straight and curved paths. Formulas are provided for determining motion given acceleration as a function of time, position, or velocity.
2) Examples of uniform and uniformly accelerated rectilinear motion, where acceleration is zero or constant, providing equations for relating position, velocity, and acceleration over time.
3) Discussion of relative motion between particles moving along the same line, noting the importance of using a common reference for time and measuring displacements relative to each other.Forces: Honors Warm-Ups
Forces: Honors Warm-UpsAmie L
Ã˝
The document contains multiple physics questions and problems related to forces, motion, friction, pulleys, and other concepts. Questions are posed about the net force on a car rounding a hill, stopping distance and force needed to stop a car, vertical motion under different applied forces, apparent weight loss in elevators, tension in pulley systems, mechanical advantage of pulleys, coefficients of static and kinetic friction, pushing and pulling boxes, acceleration of connected boxes on a pulley, speed and acceleration of a skier descending a slope, which falls faster - an elephant or feather, and other concepts involving forces, motion, and physics. Multiple choice answers are provided for some questions.Forces Warm-Ups
Forces Warm-UpsAmie L
Ã˝
The document contains multiple physics questions and problems related to forces, motion, friction, pulleys, and other concepts. Questions are posed about the net force on a car rounding a hill, stopping distance and force needed to stop a car, vertical motion under different applied forces, apparent weight in an elevator, tension in a pulley system, mechanical advantage of a pulley, coefficients of static and kinetic friction, pushing and pulling boxes, acceleration of connected boxes on a pulley, speed and acceleration of a skier descending a slope, which falls faster - an elephant or feather, forces in an accelerating elevator, acceleration of a lowered bucket, relationship between force and time to achieve a final speed, effect of initial speed on change inHonors I
Honors IAmie L
Ã˝
The document contains physics problems and explanations related to projectile motion, including questions about launch angle, horizontal launch, free fall, maximum height, time and distance calculations. Various scenarios are presented involving objects being thrown, dropped, or launched off cliffs and structures. Graphs and diagrams are included to illustrate projectile motion concepts.Chemistry Final Exam Review
Chemistry Final Exam ReviewAmie L
Ã˝
The document provides examples to test knowledge of chemistry concepts including:
1) The electron configuration of Fe2+ is [Ar]3d6. Valence electrons are in the highest energy level.
2) Francium has the largest atomic radius of the alkali metals due to atomic radius increasing down a group.
3) For a neutral atom, the number of protons and electrons are equal, determining the element's identity.Practice Redox Solutions
Practice Redox SolutionsAmie L
Ã˝
Redox solutions are used to practice balancing redox reactions. Balancing redox reactions involves identifying oxidized and reduced species and ensuring equal numbers of electrons are gained and lost. Practice with sample redox reactions will help students learn to properly balance reactions by accounting for changes in oxidation numbers.Balancing Redox HW Solutions
Balancing Redox HW SolutionsAmie L
Ã˝
This document contains 3 balanced redox reactions. The first reaction shows chlorate ions being reduced by iodide ions to form chloride ions and iodine. The second reaction shows permanganate ions being reduced by chromium ions to form manganese ions and dichromate ions. The third reaction shows tin ions being oxidized by dichromate ions to form tin ions in a higher oxidation state and chromium ions.Practice Quiz Solutions
Practice Quiz SolutionsAmie L
Ã˝
Redox solutions are used for practicing redox reactions. The slideshow provides instructions for enlarging the viewer to see the slideshow in more detail. It presents redox solutions as a tool for practicing redox reactions through visual examples and explanations shown in an interactive slideshow format.Practice Redox Solutions
Practice Redox SolutionsAmie L
Ã˝
Redox solutions are used for practicing redox reactions. The slideshow provides instructions for enlarging the viewer to see the slideshow in more detail. It presents redox solutions as a tool for practicing redox reactions through visual examples and explanations shown in an interactive slideshow format.Energy Diagrams
Energy DiagramsAmie L
Ã˝
A potential energy diagram shows the energy of reactants and products in a chemical reaction over the course of the reaction. When a catalyst is added, it creates an alternative reaction pathway that requires less energy to reach the products by lowering the activation energy of the reaction. This speeds up the reaction by making it easier for the molecules to undergo the transformation from reactants to products.Light
LightAmie L
Ã˝
The document discusses light and the electromagnetic spectrum. It asks how long it would take eyes to adjust to see an apple in the dark and provides background information on the speed of light in a vacuum and air as well as the inverse-square law of brightness and relationships between frequency, wavelength, and energy of light.Chemical Bonding
Chemical BondingAmie L
Ã˝
The document discusses different types of chemical bonding including metallic bonding, ionic bonding, and covalent bonding. Metallic bonding is described as involving delocalized electrons in a "sea of electrons" that provides properties like luster, malleability, and ductility. Ionic bonding involves the transfer of electrons and results in orderly crystal lattices and very strong bonds, forming hard but brittle solids. Covalent bonding involves the sharing of valence electrons and can be polar or nonpolar. Intermolecular forces like dipole-dipole interactions and London dispersion are also discussed.8is Enough
8is EnoughAmie L
Ã˝
The document discusses the octet rule in chemistry. It states that the octet rule says that atoms form bonds by sharing valence electrons until each atom has eight valence electrons. Atoms do this to become stable, like the noble gases. The document then asks students to determine which of several chemical formulas satisfy the octet rule and would represent stable compounds.HONC
HONCAmie L
Ã˝
This document provides an overview of molecular structures and bonding. It discusses:
1) The structural formula which shows how atoms are connected in a molecule using lines to represent covalent bonds.
2) The HONC rule which indicates how many bonds hydrogen, oxygen, nitrogen, and carbon typically form.
3) Covalent bonds which form when atoms share pairs of electrons to achieve a full outer electron shell.
4) Lewis dot structures which use dots to represent valence electrons and connect them with lines to illustrate bonding and lone pairs of electrons.Molecular Geometry
Molecular GeometryAmie L
Ã˝
Molecular geometry discusses types of bonding and electronegativity values. Different types of bonding, such as covalent and ionic bonding, determine molecular structure. Electronegativity values describe an atom's ability to attract electrons in a chemical bond.More Related Content
Viewers also liked (6)
Chapter 13 kinetics_of_particle--force_acceleration
Chapter 13 kinetics_of_particle--force_accelerationSelf-employed
Ã˝
This document discusses kinetics of particles and Newton's laws of motion. It introduces the concept of equation of motion relating the forces acting on a particle to its acceleration. Equations of motion are developed for rectangular, normal-tangential, and cylindrical coordinate systems. Examples are provided to demonstrate solving equations of motion for particles undergoing accelerated motion under various force conditions in different coordinate systems.Tutorial 1
Tutorial 1Kumutha Danasakaran
Ã˝
The document covers various topics in mechanics including statics, dynamics, kinematics, kinetics, scalars, vectors, trigonometry functions, geometry, and Newton's laws of motion. It defines concepts like particles, rigid bodies, and non-rigid bodies. It also explains the parallelogram law for adding vectors and how to use trigonometric functions and rules to solve problems involving forces and motion.Kinetics of a Particle : Force and Acceleration
Kinetics of a Particle : Force and AccelerationAbduljalil AlAbidi
Ã˝
Here are the key steps to solve this problem:
1) Draw a free body diagram of each block, showing all external forces.
2) Write the equation of motion for each block in the x and y directions: ΣFx = max, ΣFy = may
3) The tension in each cable will be the same. Substitute this into the equations of motion.
4) Solve the equations simultaneously to find the acceleration and tension.
The acceleration and tension can be determined by setting up and solving the simultaneous equations of motion for each block based on Newton's 2nd law. Friction and the coefficient of kinetic friction must be accounted for between block C and the horizontal surface.Kinetics of particle
Kinetics of particlebahria university islamabad
Ã˝
This document contains a presentation on Newton's second law of motion. The presentation topics include the relation between force, mass and acceleration, applications of Newton's second law, equations of motion, and an introduction to kinetics of particles. The document provides definitions and explanations of key concepts such as force, mass, acceleration, momentum, impulse, and kinetics. It also includes sample problems demonstrating applications of Newton's second law and equations of motion, along with step-by-step solutions. The presentation was made by Danyal Haider and Kamran Shah and covers fundamental principles of classical mechanics.Chapter 12 kinematics_of_a_particle
Chapter 12 kinematics_of_a_particleSelf-employed
Ã˝
This document discusses the kinematics of particles in rectilinear and curvilinear motion. It defines key concepts like position, displacement, velocity, and acceleration for both continuous and erratic rectilinear motion. Examples are provided to demonstrate how to construct velocity-time and acceleration-time graphs from a given position-time graph, and vice versa. The chapter then discusses general curvilinear motion, defining position, displacement, velocity, and acceleration using vector analysis since the curved path is three-dimensional. Fundamental problems and practice problems are also included.Chapter 11 kinematics of particles
Chapter 11 kinematics of particlesRogin Beldeneza
Ã˝
This document discusses kinematics concepts related to particle dynamics, including:
1) Rectilinear and curvilinear motion, describing the position, velocity, and acceleration of particles moving along straight and curved paths. Formulas are provided for determining motion given acceleration as a function of time, position, or velocity.
2) Examples of uniform and uniformly accelerated rectilinear motion, where acceleration is zero or constant, providing equations for relating position, velocity, and acceleration over time.
3) Discussion of relative motion between particles moving along the same line, noting the importance of using a common reference for time and measuring displacements relative to each other.More from Amie L (20)
Forces: Honors Warm-Ups
Forces: Honors Warm-UpsAmie L
Ã˝
The document contains multiple physics questions and problems related to forces, motion, friction, pulleys, and other concepts. Questions are posed about the net force on a car rounding a hill, stopping distance and force needed to stop a car, vertical motion under different applied forces, apparent weight loss in elevators, tension in pulley systems, mechanical advantage of pulleys, coefficients of static and kinetic friction, pushing and pulling boxes, acceleration of connected boxes on a pulley, speed and acceleration of a skier descending a slope, which falls faster - an elephant or feather, and other concepts involving forces, motion, and physics. Multiple choice answers are provided for some questions.Forces Warm-Ups
Forces Warm-UpsAmie L
Ã˝
The document contains multiple physics questions and problems related to forces, motion, friction, pulleys, and other concepts. Questions are posed about the net force on a car rounding a hill, stopping distance and force needed to stop a car, vertical motion under different applied forces, apparent weight in an elevator, tension in a pulley system, mechanical advantage of a pulley, coefficients of static and kinetic friction, pushing and pulling boxes, acceleration of connected boxes on a pulley, speed and acceleration of a skier descending a slope, which falls faster - an elephant or feather, forces in an accelerating elevator, acceleration of a lowered bucket, relationship between force and time to achieve a final speed, effect of initial speed on change inHonors I
Honors IAmie L
Ã˝
The document contains physics problems and explanations related to projectile motion, including questions about launch angle, horizontal launch, free fall, maximum height, time and distance calculations. Various scenarios are presented involving objects being thrown, dropped, or launched off cliffs and structures. Graphs and diagrams are included to illustrate projectile motion concepts.Chemistry Final Exam Review
Chemistry Final Exam ReviewAmie L
Ã˝
The document provides examples to test knowledge of chemistry concepts including:
1) The electron configuration of Fe2+ is [Ar]3d6. Valence electrons are in the highest energy level.
2) Francium has the largest atomic radius of the alkali metals due to atomic radius increasing down a group.
3) For a neutral atom, the number of protons and electrons are equal, determining the element's identity.Practice Redox Solutions
Practice Redox SolutionsAmie L
Ã˝
Redox solutions are used to practice balancing redox reactions. Balancing redox reactions involves identifying oxidized and reduced species and ensuring equal numbers of electrons are gained and lost. Practice with sample redox reactions will help students learn to properly balance reactions by accounting for changes in oxidation numbers.Balancing Redox HW Solutions
Balancing Redox HW SolutionsAmie L
Ã˝
This document contains 3 balanced redox reactions. The first reaction shows chlorate ions being reduced by iodide ions to form chloride ions and iodine. The second reaction shows permanganate ions being reduced by chromium ions to form manganese ions and dichromate ions. The third reaction shows tin ions being oxidized by dichromate ions to form tin ions in a higher oxidation state and chromium ions.Practice Quiz Solutions
Practice Quiz SolutionsAmie L
Ã˝
Redox solutions are used for practicing redox reactions. The slideshow provides instructions for enlarging the viewer to see the slideshow in more detail. It presents redox solutions as a tool for practicing redox reactions through visual examples and explanations shown in an interactive slideshow format.Practice Redox Solutions
Practice Redox SolutionsAmie L
Ã˝
Redox solutions are used for practicing redox reactions. The slideshow provides instructions for enlarging the viewer to see the slideshow in more detail. It presents redox solutions as a tool for practicing redox reactions through visual examples and explanations shown in an interactive slideshow format.Energy Diagrams
Energy DiagramsAmie L
Ã˝
A potential energy diagram shows the energy of reactants and products in a chemical reaction over the course of the reaction. When a catalyst is added, it creates an alternative reaction pathway that requires less energy to reach the products by lowering the activation energy of the reaction. This speeds up the reaction by making it easier for the molecules to undergo the transformation from reactants to products.Light
LightAmie L
Ã˝
The document discusses light and the electromagnetic spectrum. It asks how long it would take eyes to adjust to see an apple in the dark and provides background information on the speed of light in a vacuum and air as well as the inverse-square law of brightness and relationships between frequency, wavelength, and energy of light.Chemical Bonding
Chemical BondingAmie L
Ã˝
The document discusses different types of chemical bonding including metallic bonding, ionic bonding, and covalent bonding. Metallic bonding is described as involving delocalized electrons in a "sea of electrons" that provides properties like luster, malleability, and ductility. Ionic bonding involves the transfer of electrons and results in orderly crystal lattices and very strong bonds, forming hard but brittle solids. Covalent bonding involves the sharing of valence electrons and can be polar or nonpolar. Intermolecular forces like dipole-dipole interactions and London dispersion are also discussed.8is Enough
8is EnoughAmie L
Ã˝
The document discusses the octet rule in chemistry. It states that the octet rule says that atoms form bonds by sharing valence electrons until each atom has eight valence electrons. Atoms do this to become stable, like the noble gases. The document then asks students to determine which of several chemical formulas satisfy the octet rule and would represent stable compounds.HONC
HONCAmie L
Ã˝
This document provides an overview of molecular structures and bonding. It discusses:
1) The structural formula which shows how atoms are connected in a molecule using lines to represent covalent bonds.
2) The HONC rule which indicates how many bonds hydrogen, oxygen, nitrogen, and carbon typically form.
3) Covalent bonds which form when atoms share pairs of electrons to achieve a full outer electron shell.
4) Lewis dot structures which use dots to represent valence electrons and connect them with lines to illustrate bonding and lone pairs of electrons.Molecular Geometry
Molecular GeometryAmie L
Ã˝
Molecular geometry discusses types of bonding and electronegativity values. Different types of bonding, such as covalent and ionic bonding, determine molecular structure. Electronegativity values describe an atom's ability to attract electrons in a chemical bond.Connect The Dots
Connect The DotsAmie L
Ã˝
A covalent bond forms when two atoms share a pair of electrons between them. Lewis dot structures represent atoms and how their valence electrons are arranged, including any lone pairs or bonding pairs of electrons. HONC is used to indicate the number of unpaired electrons associated with hydrogen, oxygen, nitrogen, and carbon atoms.Dots Dotsand More Dots
Dots Dotsand More DotsAmie L
Ã˝
The document discusses Lewis dot structures and the octet rule for drawing molecular structures of nitrogen (N2), oxygen (O2), and fluorine (F2) gases. It explains how to draw the Lewis dot symbols for each atom and connect them to show single, double, and triple bonds. It also provides strategies for drawing Lewis dot structures, such as identifying the central atom and determining the total valence electrons. An example shows how to draw one possible structure for a molecule with the formula C2H4O2 that contains a C=O bond.Wave Interactions
Wave InteractionsAmie L
Ã˝
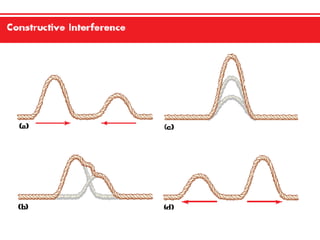
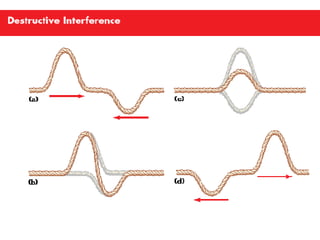
This document discusses key concepts relating to wave interactions including reflection, where a wave bounces off a surface, as well as constructive and destructive interference, which occurs when two waves meet and their peaks combine to make bigger waves or troughs combine to cancel each other out, respectively, according to the principle of superposition where the net wave is the sum of the individual waves.Electrons In Atoms
Electrons In AtomsAmie L
Ã˝
The document discusses several key concepts in atomic structure and quantum physics including:
1) Niels Bohr's early atomic model which was valid for hydrogen atoms.
2) The photoelectric effect and Max Planck's theory of quantized energy which led to Einstein's theory of photons and light behaving as particles.
3) Werner Heisenberg's uncertainty principle and Erwin Schrodinger's wave equation which helped develop the modern quantum mechanical model of the atom.Ad
Recently uploaded (20)
Test Bank for Strategic Human Resources Planning 7th Edition Belcourt
Test Bank for Strategic Human Resources Planning 7th Edition Belcourtqwjeuwwk1003
Ã˝
Test Bank for Strategic Human Resources Planning 7th Edition Belcourt
Test Bank for Strategic Human Resources Planning 7th Edition Belcourt
Test Bank for Strategic Human Resources Planning 7th Edition BelcourtCzech An Essential Grammar Bilingual James Naughton
Czech An Essential Grammar Bilingual James Naughtongkomdigywi4802
Ã˝
Czech An Essential Grammar Bilingual James Naughton
Czech An Essential Grammar Bilingual James Naughton
Czech An Essential Grammar Bilingual James NaughtonTest Bank for Marketing, 12th Edition : Lamb
Test Bank for Marketing, 12th Edition : Lambjtgpave6378
Ã˝
Test Bank for Marketing, 12th Edition : Lamb
Test Bank for Marketing, 12th Edition : Lamb
Test Bank for Marketing, 12th Edition : Lambfavole di andrea pazienza---------------
favole di andrea pazienza---------------serenacapraro
Ã˝
presentazione in power point delle favole di andrea pazienzaDormancy in Aquatic Organisms Theory Human Use and Modeling Victor R. Alekseev
Dormancy in Aquatic Organisms Theory Human Use and Modeling Victor R. Alekseevzjqcibg889
Ã˝
Dormancy in Aquatic Organisms Theory Human Use and Modeling Victor R. Alekseev
Dormancy in Aquatic Organisms Theory Human Use and Modeling Victor R. Alekseev
Dormancy in Aquatic Organisms Theory Human Use and Modeling Victor R. AlekseevContemporary Research On Business And Management Siska Noviaristanti
Contemporary Research On Business And Management Siska Noviaristantixybnhst3272
Ã˝
Contemporary Research On Business And Management Siska Noviaristanti
Contemporary Research On Business And Management Siska Noviaristanti
Contemporary Research On Business And Management Siska NoviaristantiBỘ ĐỀ THI THỬ VÀO 10 MÔN TIẾNG ANH - CÁC SỞ GIÁO DỤC THEO CHƯƠNG TRÌNH GDPT 2...
BỘ ĐỀ THI THỬ VÀO 10 MÔN TIẾNG ANH - CÁC SỞ GIÁO DỤC THEO CHƯƠNG TRÌNH GDPT 2...Nguyen Thanh Tu Collection
Ã˝
https://app.box.com/s/3rcums4t38bngk48ksg4u06ovz88b9zuNew Perspectives HTML5 and CSS3 Comprehensive 7th Edition Carey Solutions Manual
New Perspectives HTML5 and CSS3 Comprehensive 7th Edition Carey Solutions Manualgyyexgpz7373
Ã˝
New Perspectives HTML5 and CSS3 Comprehensive 7th Edition Carey Solutions Manual
New Perspectives HTML5 and CSS3 Comprehensive 7th Edition Carey Solutions Manual
New Perspectives HTML5 and CSS3 Comprehensive 7th Edition Carey Solutions ManualProject portfolio management a model for improved decision making First Editi...
Project portfolio management a model for improved decision making First Editi...hxjbigkmtq574
Ã˝
Project portfolio management a model for improved decision making First Edition Enoch
Project portfolio management a model for improved decision making First Edition Enoch
Project portfolio management a model for improved decision making First Edition Enoch(eBook PDF) Operations Management: Processes and Supply Chains 12th Global Ed...
(eBook PDF) Operations Management: Processes and Supply Chains 12th Global Ed...jdgaduqzo3480
Ã˝
(eBook PDF) Operations Management: Processes and Supply Chains 12th Global Edition
(eBook PDF) Operations Management: Processes and Supply Chains 12th Global Edition
(eBook PDF) Operations Management: Processes and Supply Chains 12th Global EditionTest Bank for Essentials of Genetics, 10th Edition, William S. Klug
Test Bank for Essentials of Genetics, 10th Edition, William S. Klugkjlzrdq0440
Ã˝
Test Bank for Essentials of Genetics, 10th Edition, William S. Klug
Test Bank for Essentials of Genetics, 10th Edition, William S. Klug
Test Bank for Essentials of Genetics, 10th Edition, William S. KlugExperiencing MIS 5th Edition Kroenke Test Bank
Experiencing MIS 5th Edition Kroenke Test Banktwyoymgar4752
Ã˝
Experiencing MIS 5th Edition Kroenke Test Bank
Experiencing MIS 5th Edition Kroenke Test Bank
Experiencing MIS 5th Edition Kroenke Test BankCatalogo WISE-ING 2025 - Seconda Competenza
Catalogo WISE-ING 2025 - Seconda CompetenzaWise Ing
Ã˝
All’interno del nostro catalogo Academy 2025, la competenza “Persona al centro” si declina in tre percorsi tra i più apprezzati da chi si occupa di leadership, formazione, customer ed employee experience e pensati non solo per manager con responsabilità di gestione ma come competenza abilitante al vivere organizzativo attuale.Economia degli intermediari finanziari 4° Edition Loris Nadotti
Economia degli intermediari finanziari 4° Edition Loris Nadottidpyutozqn7838
Ã˝
Economia degli intermediari finanziari 4° Edition Loris Nadotti
Economia degli intermediari finanziari 4° Edition Loris Nadotti
Economia degli intermediari finanziari 4° Edition Loris NadottiBỘ ĐỀ KIỂM TRA THEO TỪNG CHƯƠNG HÓA HỌC 10 - THEO CHƯƠNG TRÌNH MỚI CỦA BỘ GIÁ...
BỘ ĐỀ KIỂM TRA THEO TỪNG CHƯƠNG HÓA HỌC 10 - THEO CHƯƠNG TRÌNH MỚI CỦA BỘ GIÁ...Nguyen Thanh Tu Collection
Ã˝
https://app.box.com/s/239llqwayprfisqy4042nm9kpdipb1ogPresentation for Lesson - Lighthouse Design
Presentation for Lesson - Lighthouse DesignMax Giovagnoli
Ã˝
A show of different lighthouses from all over the world, to study their architecture and designOM 5 5th Edition Collier Solutions Manual
OM 5 5th Edition Collier Solutions Manualkrclcyny946
Ã˝
OM 5 5th Edition Collier Solutions Manual
OM 5 5th Edition Collier Solutions Manual
OM 5 5th Edition Collier Solutions ManualTỔNG HỢP 30 ĐỀ THI THAM KHẢO - ÔN THI VÀO LỚP 10 THPT CHUYÊN HÓA HỌC 2025 - C...
TỔNG HỢP 30 ĐỀ THI THAM KHẢO - ÔN THI VÀO LỚP 10 THPT CHUYÊN HÓA HỌC 2025 - C...Nguyen Thanh Tu Collection
Ã˝
https://app.box.com/s/z96an7xbk2ufrac58whgpi7hehpg1v5g30 ĐỀ THI HỌC SINH GIỎI VẬT LÍ 12 – TUYỂN CHỌN TỪ CÁC TRƯỜNG THPT TRÊN TOÀN Q...
30 ĐỀ THI HỌC SINH GIỎI VẬT LÍ 12 – TUYỂN CHỌN TỪ CÁC TRƯỜNG THPT TRÊN TOÀN Q...Nguyen Thanh Tu Collection
Ã˝
https://app.box.com/s/pua0ss89hldxz7aynkgo53lz240hc98jBỘ ĐỀ THI THỬ VÀO 10 MÔN TIẾNG ANH - CÁC SỞ GIÁO DỤC THEO CHƯƠNG TRÌNH GDPT 2...
BỘ ĐỀ THI THỬ VÀO 10 MÔN TIẾNG ANH - CÁC SỞ GIÁO DỤC THEO CHƯƠNG TRÌNH GDPT 2...Nguyen Thanh Tu Collection
Ã˝
BỘ ĐỀ KIỂM TRA THEO TỪNG CHƯƠNG HÓA HỌC 10 - THEO CHƯƠNG TRÌNH MỚI CỦA BỘ GIÁ...
BỘ ĐỀ KIỂM TRA THEO TỪNG CHƯƠNG HÓA HỌC 10 - THEO CHƯƠNG TRÌNH MỚI CỦA BỘ GIÁ...Nguyen Thanh Tu Collection
Ã˝
TỔNG HỢP 30 ĐỀ THI THAM KHẢO - ÔN THI VÀO LỚP 10 THPT CHUYÊN HÓA HỌC 2025 - C...
TỔNG HỢP 30 ĐỀ THI THAM KHẢO - ÔN THI VÀO LỚP 10 THPT CHUYÊN HÓA HỌC 2025 - C...Nguyen Thanh Tu Collection
Ã˝
30 ĐỀ THI HỌC SINH GIỎI VẬT LÍ 12 – TUYỂN CHỌN TỪ CÁC TRƯỜNG THPT TRÊN TOÀN Q...
30 ĐỀ THI HỌC SINH GIỎI VẬT LÍ 12 – TUYỂN CHỌN TỪ CÁC TRƯỜNG THPT TRÊN TOÀN Q...Nguyen Thanh Tu Collection
Ã˝
Ad
