Soil cyanobacteria.ppt
- 2. ï Cyanobacteria or Cyanoprokaryonta are oxygenic photosynthesizing prokaryotes with a wide distribution in habitat and ï range in morphology from unicellular to multicellular filamentous organisms capable of cellular differentiation.
- 3. ï any of the cyanobacteria are capable of growing on the soil and other terrestrial habitats. In addition, extant cyanobacteria also dominate the microbial populations of many extreme environments including soda lakes (Spirulina, Cyanospira), the nutrient-poor open ocean (Trichodesmium),thermal springs (Synechococcus and Mastigocladus), and the cold dry polar deserts (Chroococcidiopsis) ï They are present as cryptoendoliths in rocks in the cold dry deserts.
- 4. METHODS OF STUDYING CRUSTS: Sample collection: Crust samples are collected from the upper surface of soil stored in pre-sterilized screw cap bottles for analysis. (i) Pressing a sterile test tube into the crust, (ii) pressing a Petri dish into the crust (iii) collecting crust with a spatula and putting into a Petri dish.
- 5. ï Study of the morphology of cyanobacteria isolated: a)Crust samples can be collected from 25 positions in each site of interest and combined to homogenize by crushing and mixing. (b) To quantify cyanobacteria from each composite samples one hundred fold dilutions are made by adding 1 gm of crust to 99 ml of 0.7% saline solution and aliquots of 0.1 and 0.2 ml are spread in triplicate on two agar- solidified media Z-8
- 6. Sample collection Growth on BG11 medium
- 7. ï (c) Small fragments of soil crust may be placed in BG- 11 medium with or without combined nitrogen. They may also be placed on agar plates (1.2% w/v agar-agar in BG-11 medium). It is incubated at 25 Âą 1°C under continuous light from fluorescent tubes at an intensity 7.5 w/m2for up to 30 days.
- 9. Study of growth, pigments and macromolecules To characterize crust samples physiologically and bioche- mically their growth, pigments and macromolecules are stu- died. (a)Chlorophyll (Chla) estimation is done following the methodology of Mackinney (1941). (b)Carotenoids estimation is done following the methodology of Davis (1976). (c)Phycobiliprotein estimation is done following the methodology of Glazer and Fang (1973). (d)Scytonemin assay to be done by following the method of GarcÃa-Pichel and Castenholz (1991).
- 10. (e)Carbohydrate estimation : is done following the methodology of Herbert et al. (1971). (f)Protein estimation: is done following the methodology of Lowry et al. (1951). (g)Amino acids : are studied following the methodology of Lewis and Gonzalves (1962), Raftery and Heocha (1965). (h)Extracellular polysaccharides: if produced by crust samples, are studied following De Phillips (1998). (i)Stress protein :To detect various stress proteins, the methods of Hill et al. (1994) and Lyra et al. (2001) are followed
- 11. PHYSIOLOGICAL AND BIOCHEMICAL FEATURES OF CYANOBACTERIAL CRUST: 1- Water relation : *The various types of organisms that comprise the crust share some interesting physiological traits. *These types of organisms are referred to as (poikilohydric) *Most of them equilibrate their water content with the atmospheric humidity or soil surface moisture content.
- 12. Biological soil crust formed by cyanobacteria:
- 13. Soil crusts formed by cyanobacteria
- 14. 2-Photosynthesis : *photosynthetic oxygen evolution increased steadily and within 12 to 24 h of wetting 3- Mineral nutrition: *presence of soil crusts increases surrounding soil N by up to 200%. *Crust organisms show negative influences on ion up-take by seed plants for iron (Fe), manganese (Mn), sodium (Na), phosphorus (P) and zinc (Zn). *Crust organisms secrete extracellular polymers. They increase soil polysaccharides and total carbon by up to 300% *Cyanobacteria secrete metal chelators such as siderochromes that maintain metals in a biologically available form
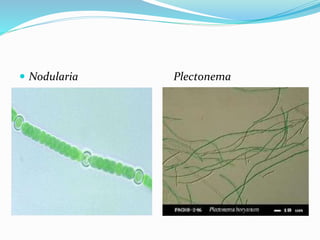
- 15. 4-N fixation: ï N fixed by the cyanobacteria and cyanolichens found in the biological soil crusts can be the dominant source of N. ï Most cyanobacterial N fixation takes place in heterocysts. Heterocystic genera that commonly occur in soil crusts include Anabaena, Calothrix, Cylindrospermum, Hapalosiphon, Nodularia, Nostoc, Plectonema , Schizothrix and Scytonema
- 17. 5- UV-absorbing pigment : *Many members of cyanobacteria contain UV-A/B absorbing MAA (Mycosporine-like amino acids â Mycosporines comprise a diverse family of small molecular weight, colourless, water-soluble secondary metabolites) *MAA synthesis is induced by osmotic and UV stress. In the firm sheaths of many colonial and filamentous cyanobacteria yellow, red and blue pigments may accumulate. The typical yellow brown pigment has been characterized as a UV- absorbing protective pigment scytonemin
- 18. Cyanobacterial species found in soil crusts: Anabeana Calothrix
- 19. Cyanobacterial species found in soil crusts: Cylindrospermum Hapalosiphon
- 21. Cyanobacterial species found in soil crusts: Schizothrix Scytonema
- 22. IMPORTANCE OF CYANOBACTERIAL SOIL- CRUSTS 1- Carbon-fixation 2- N-fixation 3-Role in reduction of soil, wind and water erosion and improvement of soil quality 4-Retention of soil moisture , water relation and reduction of weed growth 5- Acting ecological indicators 6-Role in ecological rehabilitation 7-Production of extra-cellular polysaccharides
- 23. 8- Secretion of exopolymers and metal chelators 9-Influence on mineral uptake by associated vascular plants 10-Creation of artificial soil crust 11-Effects on germination of vascular plants 12-Conservation of biological soil crust
- 24. References: ï Bhattacharya,S., Rath,J and Ray,S. (2012) Composition, Basic Features and Distribution of Cyanobacteria in Soil Crusts,Dynamic Biochemistry, Process Biotechnology and Molecular Biology 6 (1-12)