Solid State.pptx
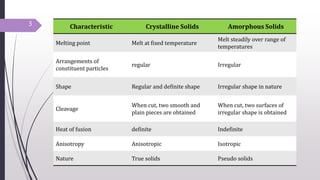
- 3. 3 Characteristic Crystalline Solids Amorphous Solids Melting point Melt at fixed temperature Melt steadily over range of temperatures Arrangements of constituent particles regular Irregular Shape Regular and definite shape Irregular shape in nature Cleavage When cut, two smooth and plain pieces are obtained When cut, two surfaces of irregular shape is obtained Heat of fusion definite Indefinite Anisotropy Anisotropic Isotropic Nature True solids Pseudo solids
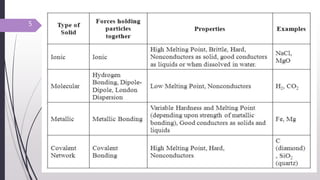
- 4. Solid state 1. In a solid, the particles (atoms, molecules or ions) are closely packed together. 2. The forces between the particles are strong so that the particles cannot eely but can only vibrate. 3. Thus a solid has a stable, definite shape and a definite volume. Their shape can be changed only by application of force. TYPES OF SOLIDS: 1. Following are the different types of solids: Crystalline solids : It exists as small crystals, each crystal having a characteristic geometrical shape, in a crystal, the atoms, molecules or ions. are arranged in a regular repeating three dimensional pattern called the crystal lattice. 4
- 5. 5
- 6. Solid state Amorphous solids : ï It has atoms, molecules or ions arranged at random and lacks the ordered crystalline lattice. ï In their disordered structure, amorphous solids resemble liquids. ï Crystalline substances are said to be anisotropic (i.e. the magnitude of physical property varies with directions) whereas amorphous solids are said to be isotropic (i.e. the magnitude of physical property remains the same in all directions). 6
- 7. Solid state Depending upon the nature of forces acting the solids can be divided into four types: (i) Covalent Solids: In these solids the constituent units are attached by covalent linkages. Depending on arrangement, these can be divided into isotropic and anisotropic substances. Isotropic Substances: These systems show uniform velocity of light in all directions. Amorphous substance exhibit this property. Other parameters like refractive index, thermal and electrical conductivities are also uniform in all directions. Eg : Water, glass 7
- 8. Solid state Anisotropic Substances: ï The velocity of light is not uniform in all directions. ï When a ray enters anisotropic substance it splits up into two separate components which travel with different velocities. ï The other physical properties which are mentioned in isotropic substance also vary in anisotropic substances. ï Eg: Silver iodide. 8
- 9. PROPERTIES OF Crystals: Crystal habit: The external shape of the crystal is called as the crystal habit. The plane surfaces of the crystal are called faces and angles between the faces are called interfacial angles. The interfacial angles for a given crystalline substance are always the same. Crystal symmetry: This is an important property of crystals. There are three types of symmetry elements associated with a crystal. These are called elements of symmetry. 9
- 10. PROPERTIES OF Crystals: ïą Plane of symmetry: A crystal is said to have plane of symmetry if it can be divided by an imaginary line into equal parts, each of which is exact mirror image of the other. ïą Axis of symmetry: It is an imaginary line drawn through the crystal such that during rotation of crystal through 360°,the crystal looks the same more than once. ïą Centre of symmetry : It is a point at the centre of the crystal so that any line drawn through it will meet the surface or the crystal at equal distance on either side. 10
- 11. PROPERTIES OF Crystals: Crystal structure: The particles (atoms, molecules or ions) in crystals are highly ordered and they are arranged in a regular pattern that extend in all directions. The overall arrangement of particles in a crystal is called the crystal lattice, space lattice or lattice. 11
- 12. 12
- 13. 13
- 14. 14
- 15. Methods of crystal analysis ï§ When a beam of X-rays is allowed to pass through crystal lattice, a large number of images of different intensities are formed. ï§ A crystal lattice is considered to be made up of regular planes which are separated by equal distance. ï§ Since the wavelength of X-ray is comparable to the interatomic distances, Laue suggested that crystal can act as grating to X-rays. ï§ So, if the diffracted waves are in the same phase, they reinforce each other and a series of bright spots are produced on a photographic plate placed in their path as shown in Fig. 15
- 16. Methods of crystal analysis ï§ On the other hand, if the diffracted waves are out of phase, interference will result, and dark spots are caused on the photographic plate. ï§ From the overall diffraction patterns produced by a crystal, we can get the detailed information regarding the position of particles in the crystal. 16
- 17. Bragg's equation ï§ In 1913 W.L. Bragg made a successful attempt in determining the interatomic distance in a crystal lattice using X-rays. The study of crystal structure with the help of X-rays is called X-ray crystallography. ï§ He showed that: ï§ X-rays obey laws of reflection ï§ The extra distance travelled by the second ray [P] is an integral number of wavelength ðð = ðð ðððð― 17
- 18. Bragg's equation ðÎŧ=2ð ð ððð ï§ For a given set of lattice planes d and A are fixed. ï§ d - distance between ï§ Îŧ - wavelength of x-rays ï§ Îļ - Angle at which the X-rays strike the crystal ï§ n - order of reflection. 18
- 19. Deviation of Bragg's equation ï§ As shown in figure a beam of X-ray is following on the crystal surface. ï§ The 2 successive planes of crystal separated by distance, d. ï§ Let X-ray of wavelength Îŧ strike the force first plane at an angle Îļ same rays are reflected at the same plane. And some will penetrate and get reflected from the second plane. ï§ These rays will be in force with those reflected from first plane. If extra distance (CB+BD) travelled by them is equal to integral no. n of the wavelengths. ï§ i.e. n Îŧ = CB+BD -----------(1) 19
- 20. Deviation of Bragg's equation From the geometry we know that CB = BD = AB sin Îļ ---------(2) From (1) and (2) ðÎŧ=2AB ð ððð ðÎŧ=2ð ð ððð This is known as Braggâs Equation 20
- 21. Measurement of diffraction angle Îļ 1. Rotating crystal method (Bragg 1913) 2. The powder method ( Debye & Scherrer 1916) 21
- 23. Rotating crystal method ï A beam of x-rays of known Îŧ falls on a face of the crystal mounted on a graduated turn table. ï The diffracted rays pass into the ionisation chamber of the recorder. ï Here they ionise the air and a current flows between the chamber wall and the electrode inserted in it which is connected to an electrometer. ï The electrometer reading is proportional to the intensity of x- rays 23
- 24. Rotating crystal method ï As the recorder along with the crystal is rotated, the angles of maximum intensity are noted on the scale. ï Thus values of Îļ for n= 1,2,3 etc are used to calculate the distance d between the lattice planes parallel to the face of the crystal 24
- 25. The powder method ï In this method, the crystalline material contained in a capillary tube is placed in the camera containing a film strip ï The sample is rotated by means of a motor ï The x-rays passes through the gap between the ends of the film. ï The powdered sample contains small crystals arranged in all orientations. ï Some will reflect x-rays from each lattice plane at sometimes. ï The reflected X-rays make an angle of 2 Îļ with the incident X-ray. ï From the geometry of camera, Îļ can be calculated for different crystal planes. 25
- 27. THANK YOU 27















![Bragg's equation
ï§ In 1913 W.L. Bragg made a successful attempt in determining the
interatomic distance in a crystal lattice using X-rays. The study of crystal
structure with the help of X-rays is called X-ray crystallography.
ï§ He showed that:
ï§ X-rays obey laws of reflection
ï§ The extra distance travelled by the second ray [P] is an integral number of
wavelength
ðð = ðð
ðððð―
17](https://image.slidesharecdn.com/solidstate-220910064604-955d8a07/85/Solid-State-pptx-17-320.jpg)









