Solvent extraction of salvia leaves by decantation using the solvent methanol
- 1. Solvent extraction of Salvia leaves by decantation using the solvent MeOH 6th November 2014 Presented by: Bhagea Ritesh Buctowar Rouksaar C├®cile Christabelle Ghoorbin Keshavi Nazeer Huda
- 2. Contents ŌŚÅ Introduction ŌŚÅ Literature review ŌŚÅ Methodology of decantation followed by rotary evaporator ŌŚÅ Yield of extract ŌŚÅ Phytochemical screening methods ŌŚÅ Result ŌŚÅ Discussion ŌŚÅ Conclusion
- 3. Introduction Solvent Extraction ŌŚÅ Method employed to separate substances based on their solubility. ŌŚÅ Used in the extraction of phytochemicals from plants. Phytochemicals are: ŌŚÅ Non-nutritive plants chemicals, ŌŚÅ Not crucial to sustain human life but are beneficial, ŌŚÅ Some common examples are lycopene in tomatoes and isoflavones in soy, ŌŚÅ Have beneficial effects such as: ŌŚŗ Antioxidant, Antimicrobial, Anticancer, etc ŌŚÅ Can be obtained from a variety of plants like: ŌŚŗ medicinal ones, herbs, fruits/nuts.
- 4. Introduction Salvia officinalis ŌŚÅ Commonly known as sage, ŌŚÅ Belongs to Lamiaceae (Labiatae) family, ŌŚÅ Perennial herbaceous plant, ŌŚÅ Can reach 50 cm in height, ŌŚÅ Used in food as herbs and in beverages such as tea, ŌŚÅ Used in the cosmetics industry. Salvia leaves Salvia flower Salvia tea
- 5. Introduction Salvia officinalis ŌŚÅ Used in culinary and medicinal preparations, ŌŚÅ Salvia species have many pharmaceutical properties ŌŚŗ Antibacterial, ŌŚŗ Antiviral, ŌŚŗ Antioxidative, ŌŚŗ Anticancer and many others ŌŚÅ Contains large amounts of flavonoids ŌŚÅ Suitable for further studies in Phytochemistry Dried Salvia leaves
- 6. Literature review ŌŚÅ According to Martins et al. (2014), S.officinalis has antioxidant and antifungal properties. ŌŚÅ Made use of decoctions, infusions and methanol/water extracts ŌŚÅ Testing for phenolic compounds ŌŚÅ 21 compounds were detected by HPLC ŌŚŗ 10 phenolic acids and 11 flavonoids ŌŚÅ Some compounds were: ŌŚŗ Luteolin digluceronide ŌŚŗ Sagecoumarin ŌŚŗ Caffeic acid ŌåÆ ŌŚŗ Luteolin-7-O-glucoside ŌŚÅ More compounds detected in decoctions than water/ethanol and infusion
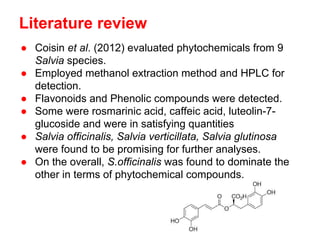
- 7. Literature review ŌŚÅ Coisin et al. (2012) evaluated phytochemicals from 9 Salvia species. ŌŚÅ Employed methanol extraction method and HPLC for detection. ŌŚÅ Flavonoids and Phenolic compounds were detected. ŌŚÅ Some were rosmarinic acid, caffeic acid, luteolin-7- glucoside and were in satisfying quantities ŌŚÅ Salvia officinalis, Salvia verticillata, Salvia glutinosa were found to be promising for further analyses. ŌŚÅ On the overall, S.officinalis was found to dominate the other in terms of phytochemical compounds.
- 8. Methodology - Decantation Extraction of non-volatile secondary metabolites by decantation: ŌŚÅ 28g of fresh Salvia leaves were measured ŌŚÅ 250 ml of methanol was measured into a conical flask ŌŚÅ The leaves were added to the flask, sealed with a cotton wool and aluminium foil and wrapped in newspaper ŌŚÅ The flask was then put on a shaker for 48 hours
- 9. Methodology ŌŚÅ Afterwards, the laboratory vacuum pump machine was used to remove unwanted solids from the solvent ŌŚŗ Tissue paper was put in the porous funnel and the solution was passed through ŌŚŗ To the filtrate obtained, MgSO4 powder was added to absorb any water present ŌŚŗ The powder was continuously added until no more agglutination occurred ŌŚŗ Filtration was again carried out using filter paper instead Hooshna using the lab vacuum filter pump
- 10. Methodology ŌŚÅ The solution was then concentrated using the rotary evaporator machine. ŌŚŗ Mass of empty rotary round bottom flask was taken ŌŚŗ Water bath was set at 65o C, pressure was closed in the condenser, pump machine was put on and water was allowed into the condenser. Rotary evaporator machine
- 11. Methodology ŌŚÅ The process was completed when all solvent got evaporated into the collecting flask and when a concentrated extract was formed ŌŚŗ Mass of rotary round bottom flask + extract were measured ŌŚÅ The extract was removed using a pipette and 10 ml of solvent was added again to dissolve any extract left. This was the diluted extract.
- 12. Calculation of yield From the 5 groups: Total amount of methanol solvent = ~1050ml Total amount of Salvia leaves = ~110g Mass of empty rotary round bottom flask = 328.4g Mass of flask + extract = 375g Mass of extract = (375 - 328.4) = 46.6g Yield of non volatile extract = Weight of extract x 100 % Weight of sample used Yield = (46.6/110) x 100 = 42.37 %
- 13. Phytochemical screening There are 2 ways to do phytochemical analysis: by test-tube tests and by TLC methods. 1. Coumarins - Conc. ammonia solution added to crude extract - A smear of this sol. placed on a microscope slide - Viewed under UV light at 366nm 2. Steroid/Terpenes - Extract separated on TLC in solvent system (9:1 Chloroform/Methanol), (40:10)ml respectively - Plate developed by spraying of LB reagent and dried
- 14. Phytochemical Screening 3. Tannins - Extract washed with petroleum ether and filtered - Equal amount of freshly prepared ferric chloride and potassium hexacyanoferrate (dropwise) added to the filtrate 4. Phenols - Extract separated on TLC in solvent system (4:1 Chloroform/Methanol) - Plate developed by spraying of Folin reagent
- 15. Phytochemical Screening 5. Alkaloids - Extract separated on TLC in solvent system (9:2:8:1 Ether/Methanol/Acetone/Ammonia) - Plate developed by spraying of Dragendorff reagent 6. Saponins - 0.5g of dried crushed leaves put in water bath (100o C) for 5 min - Cooled and shaken vigorously Spraying Dragendorff on silica gel plate
- 16. Phytochemical Screening 7. Anthraquinones - 10 drops extract dissolved in 10 drops water - Sol. filtered and extracted with benzene - Ammonia was then added and shaken 8. Leucoanthocyanins and flavonols - Extract washed with petroleum ether to extract all pigments - Ethanol added and filtered into 2 test tubes - Conc. HCl added in both test tubes - To one, Mg turnings added and allowed to stand for 10min - The other, placed in hot water bath and allowed to stand for 30 min
- 17. Results Table 1: Methanol extracts of leaves from Salvia officinalis Compounds Salvia Plant Coumarins No result Steroids/ terpenes - Tannins + Phenols + Alkaloids - Saponins - Anthraquinones + Leucoanthocyanins and flavonols -
- 18. TLC result for Steroid/Terpenes The positive result for presence of steroid/terpenes is a blue colour after spraying with LB reagent. The methanol extract (M) on the left, does not show any positive result. Thus, steroid/terpenes were not present. Figure 1: Thin Layer Chromatography of methanolic extracts of leaves from Salvia officinalis.
- 19. TLC result for phenols The positive result for presence of phenols is a colour of blue to grey after spraying of Folin reagent. The methanol extract (M) on the left, shows positive result, being grey. Thus, the presence of phenols is confirmed. Figure 2: Thin Layer Chromatography of methanolic extracts of leaves from Salvia officinalis.
- 20. TLC result for alkaloids The positive result for presence of alkaloids when spraying with Dragendorff is orange-brown spots on a yellow background. The methanol extract (M) on the left, does not show any positive result. Thus, alkaloids were not present. Figure 3: Thin Layer Chromatography of methanolic extracts of leaves from Salvia officinalis.
- 21. Discussion ŌŚÅ Polarity is an ability of a molecule to form strong bonds with other polar molecules (Barwick et al. 1997) ŌŚÅ The solvent methanol which was used is a polar organic solvent having a hydroxyl group that attracts polar molecules. ŌŚÅ But, it can also dissolve the non-polar ones, although to a lower conc. ŌŚÅ The phytochemical screening of Salvia gave positive results for tannins, phenols and anthraquinones; implying the presence of all 3.
- 22. Discussion ŌŚÅ According to a study (Ramu et al., 2012), the presence of terpenoids and steroids as well as tannins and flavonoids yielded a positive result for the Salvia officinalis. However, it is not the case in these experiments carried out. ŌŚÅ According to another study (Mattalib and Naqishbandi, 2012), the presence of flavonoids, saponins and tannins were positive. ŌŚÅ According to a study from Coisin et al. 2012,, flavonoids and polyphenolic compounds were found present in all Salvia species.
- 23. Discussion ŌŚÅ According to a study by Muria et al. 2002, 11 abietane diterpenoids, 3 apianane terpenoids, 1 anthraquinone, and 8 flavonoids were isolated from the Salvia officinalis plant. Ō×ö From the studies just mentioned, it can be concluded that the 3 compounds obtained (tannins, phenols and anthraquinones) from the decantation with methanol, are really present in the plant. However, other compounds such as terpenoids, steroids, flavonoids and saponins were not obtained. Ō×ö This would lead to speculate that decantation is not a suitable method and also that methanol might not have extracted some non-polar compounds
- 24. Conclusion The Salvia officinalis has a lot of potential in terms of research for phytochemicals. It has been shown that a large variety of flavonoids and phenolic compounds are available in this plant. However, decantation has not shown as being a good method for extraction. For much detailed or in depth research, much precise equipments must be used such as HPLC or mass spectrometry.
- 25. References ŌŚÅ British Herbal Medicine Association. (1971). British herbal pharmacopoeia. Nr. Keighley: British Herbal Medicine Association. ŌŚÅ COISIN, M., NECULA, R., GRIGORA┼×, V., GILLE, E., ROSENHECH, E., and ZAMFIRACHE, M. M. (2012). PHYTOCHEMICAL EVALUATION OF SOME SALVIA SPECIES FROM ROMANIAN FLORA. Analele Stiintifice Ale Universitatii Alexandru Ioan Cuza Din Iasi. Sectiunea II A, Biologie Vegetala 58(1). Retrieved from http: //www.bio.uaic.ro/publicatii/anale_vegetala/issue/2012F1/05-2012F1.pdf ŌŚÅ Kontogianni, V. G., Tomic, G., Nikolic, I., Nerantzaki, A. A., Sayyad, N., Stosic- Grujicic, S. and Tzakos, A. G. (2013). Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti- proliferative activity. Food Chemistry 136(1), 120ŌĆō129. ŌŚÅ Martins, N., Barros, L., Santos-Buelga, C., Henriques, M., Silva, S., and Ferreira, I. C. F. R. (2015). Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chemistry 170, 378ŌĆō385. ŌŚÅ Muira, K., Kikuzaki, H. and Nakatani N. (2002). Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. Journal of Agricultural Food Chemistry, 50(7), pp 1845-1851. ŌŚÅ Muttalib, L.Y. and Naqishbandi, A.M. (2012). Antibacterial and phytochemical study of Iraqi of Salvia officinalis leaves extracts. Iraqi Journal of Pharmaceutical Science, 21(1).