Stabilization of emulsion via electrostatic
- 2. What is an Emulsion?  A dispersion of one liquid in a second, immiscible liquid.  There are two phases: Dispersed phase and continuous phase.  Two distinct type of emulsion: O/W and W/O.  O/W – Oil is the dispersed phase while water is the continuous phase. Example: Mayonnaise, milk.  W/O – Water is the dispersed phase while oil is the continuous phase. Example: Butter.
- 3. Intermolecular Forces.  There are 2 intermolecular forces which affect the stability of dispersed system.  Van der Waals force is an attractive force, which tends to destabilize the emulsion.  Electrostatic force is a repulsive force, which gives stability to the emulsion.  When the total attractive forces > total repulsive forces, the emulsion is unstable.  When total repulsive forces > total attractive forces, the emulsion is stable.  Often, emulsions are inherently unstable.  The small suspended droplets first coalesce into less stable, larger droplets. This coalescence then continues until the emulsion breaks into two layers.
- 4.  This phenomenon can occur over a relatively short period of time.  If a long emulsion lifetime is required, therefore, a stabilizer needs to be added.  This stabilizer is usually a surfactant.
- 5. Electrostatic Forces.  Electrostatic stabilization of colloids: A mechanism in which the attraction Van der Waals forces are counterbalanced by the repulsive Coulomb forces acting between negatively charged colloidal particles.
- 6. Electrical Double Layer.  A phenomenon, which plays a fundamental role in the mechanism of the electrostatic stabilization of colloids.  It has a combination of charged surface.  Unequal distribution of co-ions and counter-ions near the surface.  Colloidal particles gain negative electric charge when negatively charged ions of the dispersion medium are adsorbed on the particles surface.  A negatively charged particle attracts the positive counter-ions surrounding the particle.  Electric Double Layer is the layer surrounding a particle of the dispersed phase and including the ions adsorbed on the particle surface and a film of the counter-charged dispersion medium
- 8.  The Electric Double Layer is electrically neutral.  It consists of three parts: Surface charge - charged ions (commonly negative) adsorbed on the particle surface.  Stern layer – counter-ions (charged opposite to the surface charge) attracted to the particle surface and closely attached to it by the electrostatic force.  Diffuse layer - a film of the dispersion medium (solvent) adjacent to the particle. Diffuse layer contains free ions with a higher concentration of the counter-ions. The ions of the diffuse layer are affected by the electrostatic force of the charged particle.  It is sensitive to electrolytes and temperature.
- 9.  The electrical potential within the Electric Double Layer has the maximum value on the particle surface (Stern layer).  The potential drops with the increase of distance from the surface and reaches 0 at the boundary of the Electric Double Layer.  When a colloidal particle moves in the dispersion medium, a layer of the surrounding liquid remains attached to the particle.  The boundary of this layer is called slipping plane (shear plane).  The value of the electric potential at the slipping plane is called Zeta potential, which is very important parameter in the theory of interaction of colloidal particles.
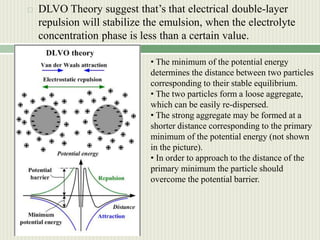
- 11. DLVO theory.  Deryagin, Landau, Vewey and Overbeek developed a theory of the stability of colloidal systems (DLVO theory) in the 1940s.  Assumptions of DLVO theory: 1. Dispersion is dilute. 2. Only two forces act on the dispersed particles: Van der Waals force and electrostatic force. 3. The electric charge and other properties are uniformly distributed over the solid surface. 4. The distribution of the ions is determined by the electrostatic force, Brownian motion and entropic dispersion.  The theory states that the colloidal stability is determined by the potential energy of the particles (VT) summarizing two parts: potential energy of the attractive interaction due to van der Waals force VA and potential energy of the repulsive electrostatic interaction VR: VT = VA + VR
- 12.  DLVO Theory suggest that’s that electrical double-layer repulsion will stabilize the emulsion, when the electrolyte concentration phase is less than a certain value. • The minimum of the potential energy determines the distance between two particles corresponding to their stable equilibrium. • The two particles form a loose aggregate, which can be easily re-dispersed. • The strong aggregate may be formed at a shorter distance corresponding to the primary minimum of the potential energy (not shown in the picture). • In order to approach to the distance of the primary minimum the particle should overcome the potential barrier.
- 13. Zeta-Potential.  It is the electrical potential at the hydrodynamic plane of shear.  The particles interact according to the magnitude of the zeta potential, not their surface charge.  Zeta potential tells us about the effectiveness of the surface charge.  For electrically stabilized dispersion, the higher the value of zeta potential, the more stable the dispersion is likely to be.
- 14.  Stability dividing line is generally considered to be ± 30mV.  Particles with the zeta potential more than +30mV or less than -30mV formed a stable dispersion.  Small changes in pH or concentration of ions (ionic strength) can lead to dramatic changes in the zeta potential.
- 15. Effect of pH on Zeta Potential.
- 16. Potential Energy Curve for Stable and Unstable Emulsion.
- 18. References. 1. Proceedings of the Symposium on the Electrochemical Double Layer, By Carol Korzeniewski, B. E. Conway. 2. Colloids in agrochemicals: colloids and interface science, Volume 5, By Tharwat F. Tadros
- 19. Group Members: 1. Ng Mong Ling 104444 2. See Kah Ling 3. Tan Woon Li 104502 4. Yeap Chiao Li 104512
- 20. - THE END - THANK YOU