Static gk chemistry
- 1. Chemistry Element: It cannot be split into simpler substances, e.g., iron, sulphur, oxygen, gold. There are about 106 elements. Compound: It can be split into simpler substances and is formed by the union of two or more elements in definite proportions by weight, e.g. 1. Water can be split into hydrogen and oxygen. 2. Iron sulphide is split into iron and sulphur. 3. Chalk is made of calcium, carbon and oxygen. 4. Carbondixoide is made up of carbon and oxygen. Mixture: It is one in which two or more substances are mixed together in any ratio without altering their properties. 1. Sand and salt 2. Sugar and water 3. Air is a mixture of nitrogen and oxygen 4. Gun-powder is a mixture of nitre, charcoal and sulphur Hard and Soft Water: Hard water is that water which does not produce lather with soap easily. Soft water produces lather with soap very easily. There is two kinds of hardness: - (i) Temporary (ii) Permanent. ï· Temporary is due to bicarbonates of calcium and magnesium. Can be removed by (i) boiling, (ii) addition of lime. ï· Permanent is due to the sulphates and chlorides of calcium and magnesium. Permanent hardness can be removed by (I) addition of washing soda, or (ii) by distillation. Alloy: Is a mixture of two or more metals in a certain proportion. Alloys are made to impart certain special properties to the metals in question. For example, stainless steel is made using Iron, Manganese, Carbon etc. to give it high strength, elasticity etc which is not found in Iron. Amalgam: any alloy containing Mercury as an essential component is known as amalgam.
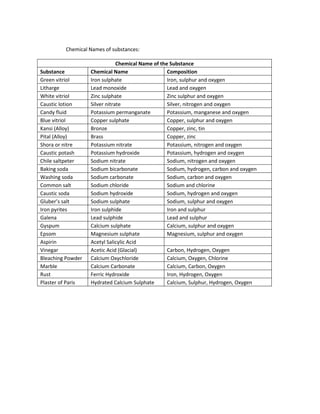
- 2. Chemical Names of substances: Chemical Name of the Substance Substance Chemical Name Composition Green vitriol Iron sulphate Iron, sulphur and oxygen Litharge Lead monoxide Lead and oxygen White vitriol Zinc sulphate Zinc sulphur and oxygen Caustic lotion Silver nitrate Silver, nitrogen and oxygen Candy fluid Potassium permanganate Potassium, manganese and oxygen Blue vitriol Copper sulphate Copper, sulphur and oxygen Kansi (Alloy) Bronze Copper, zinc, tin Pital (Alloy) Brass Copper, zinc Shora or nitre Potassium nitrate Potassium, nitrogen and oxygen Caustic potash Potassium hydroxide Potassium, hydrogen and oxygen Chile saltpeter Sodium nitrate Sodium, nitrogen and oxygen Baking soda Sodium bicarbonate Sodium, hydrogen, carbon and oxygen Washing soda Sodium carbonate Sodium, carbon and oxygen Common salt Sodium chloride Sodium and chlorine Caustic soda Sodium hydroxide Sodium, hydrogen and oxygen Gluberâs salt Sodium sulphate Sodium, sulphur and oxygen Iron pyrites Iron sulphide Iron and sulphur Galena Lead sulphide Lead and sulphur Gyspum Calcium sulphate Calcium, sulphur and oxygen Epsom Magnesium sulphate Magnesium, sulphur and oxygen Aspirin Acetyl Salicylic Acid Vinegar Acetic Acid (Glacial) Carbon, Hydrogen, Oxygen Bleaching Powder Calcium Oxychloride Calcium, Oxygen, Chlorine Marble Calcium Carbonate Calcium, Carbon, Oxygen Rust Ferric Hydroxide Iron, Hydrogen, Oxygen Plaster of Paris Hydrated Calcium Sulphate Calcium, Sulphur, Hydrogen, Oxygen