stuvite formation (1).pptx-lechate treatde onet
- 1. Waste to Energy Project
- 2. Background of leachate ŌĆó Incineration Leachate is high concentration of organic pollutants. ŌĆó Commonly, the COD concentration is around 40000-80000 mg/L. ŌĆó Leachate is high concentration of ammonical nitrogen around 1000-2000 mg/L. ŌĆó Leachate has high concentration of heavy metal ions and salts, which come from raw municipal waste. ŌĆó Low PH and contain large amount of organic acids. ŌĆó High concentration of SS.
- 4. Without Coagulation?? ŌĆó A high contents of calcium in the influent of an anaerobic reactor can lead to excessive precipitation of CaCO3. ŌĆó Scaling of reactor walls and effluent pipes ŌĆó Loss of buffer capacity. ŌĆó Loss of specific methanogenic activity. ŌĆó Space occupation by inorganic precipitates.
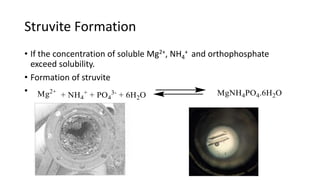
- 5. Struvite Formation ŌĆó If the concentration of soluble Mg2+, NH4 + and orthophosphate exceed solubility. ŌĆó Formation of struvite ŌĆó
- 7. Coagulation Process ŌĆó The ferrous sulfate had been used in water and wastewater treatment. ŌĆó It also has been used for the removal of nitrogen, chemical phosphorus removal. ŌĆó ferrous sulfate is added after pH adjustment. ŌĆó The chemical reactions in ferrous coagulation treatment.
- 8. Thank You