Suzuki coupling reaction
- 2. 2Department of chemistry, University of Agriculture, Faisalabad
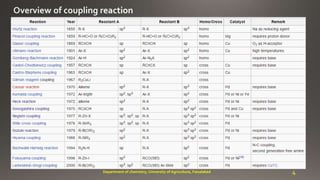
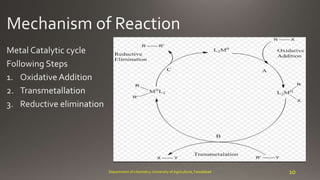
- 3. carbon-carbon bond 3Department of chemistry, University of Agriculture, Faisalabad R-X + M-R R-R + MX
- 4. 4Department of chemistry, University of Agriculture, Faisalabad
- 5. Richard F. Heck Ei-ichi Negishi Akira Suzuki Akira SuzukiEi-ichi NegishiRichard F. Heck Previously in this area: The Grignard reaction (1912), the Diels-Alder reaction (1950), the Wittig reaction (1979), and olefin metathesis (2005). 5Department of chemistry, University of Agriculture, Faisalabad
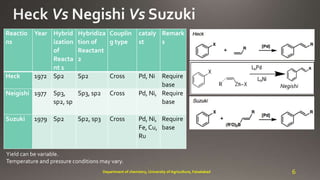
- 6. Reactio ns Year Hybrid ization of Reacta nt 1 Hybridiza tion of Reactant 2 Couplin g type cataly st Remark s Heck 1972 Sp2 Sp2 Cross Pd, Ni Require base Neigishi 1977 Sp3, sp2, sp Sp3, sp2 Cross Pd, Ni, Require base Suzuki 1979 Sp2 Sp2, sp3 Cross Pd, Ni, Fe, Cu, Ru Require base Yield can be variable. Temperature and pressure conditions may vary. Negishi 6Department of chemistry, University of Agriculture, Faisalabad R
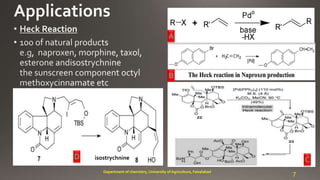
- 7. A B 7Department of chemistry, University of Agriculture, Faisalabad CD isostrychnine
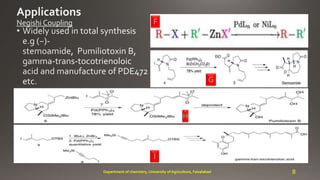
- 8. F G H I 8Department of chemistry, University of Agriculture, Faisalabad
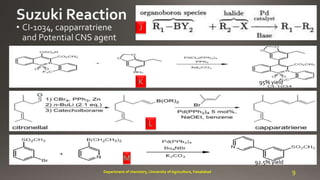
- 9. J K L M 95% yield 92.5% yield 9Department of chemistry, University of Agriculture, Faisalabad
- 10. Department of chemistry, University of Agriculture, Faisalabad 10
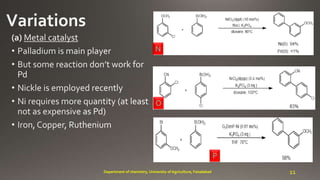
- 11. N O P 11Department of chemistry, University of Agriculture, Faisalabad
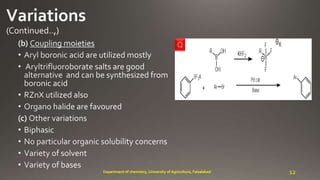
- 12. Q 12Department of chemistry, University of Agriculture, Faisalabad
- 13. 13Department of chemistry, University of Agriculture, Faisalabad
- 14. References B?ckvall, J. (2010). "Palladium-CatalyzedCross Couplings inOrganic Synthesis: Scientific Background on the Nobel Prize in Chemistry 2010."The Royal Swedish Academy of Sciences. Das, P. andW. Linert (2016). "Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki¨CMiyaura reaction."CoordinationChemistry Reviews 311: 1-23. Gujral, S. S., et al. (2012). "Suzuki Cross Coupling Reaction-A Review." Indo Global J. Pharm. Sci 2: 351- 367. Maluenda, I. and O. Navarro (2015). "Recent Developments in the Suzuki-Miyaura Reaction: 2010¨C 2014." Molecules 20(5): 7528-7557. Suzuki, A. (1999). "Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995¨C1998." Journal of OrganometallicChemistry 576(1): 147-168. Is there any question?Thank you! 14