Suzuki reaction
- 1. SUZUKI REACTION SUZUKI MIYAURA REACTION SUZUKI COUPLING REACTION SUBMITTED BY : RINSHANA FATHIMA ABDUL AZEEZ FIRST YEAR M.PHARM PHARMACEUTICAL CHEMISTRY AL SHIFA COLLEGE OF PHARMACY 1
- 2. Suzuki reaction is the Pd catalysed cross coupling reaction[1] between the boronic / organoboronic acids / organoboranes with organic halides[2], triflates, etc under basic conditions[3] which leads to the formation of carbon carbon single bonds.[4] ïThis reaction is commonly used for synthesising biaryl compounds. 2
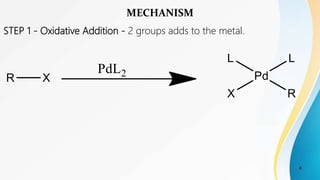
- 4. STEP 1 - Oxidative Addition - 2 groups adds to the metal. 4 MECHANISM
- 5. STEP 2 - Hydroxide attacks Pd, kicking off X 5
- 6. STEP 3 - Lewis base (OHïž) attacks Lewis acid (B) 6 The Organo Boron has only 3 bonds around it and it is sp2 hybrised with an empty p orbital, making it a very good lewis acid.
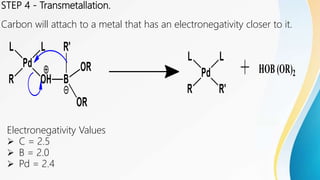
- 7. STEP 4 - Transmetallation. Carbon will attach to a metal that has an electronegativity closer to it. Electronegativity Values ï C = 2.5 ï B = 2.0 ï Pd = 2.4
- 8. STEP 5 - Reductive Elimination - 2 groups are removed from a metal. 8
- 9. ADVANTAGES âĒ Mild reaction conditions. (From 20-80â) âĒ Commercial availability of many boronic acids. âĒ The inorganic by-products are easily removed from the reaction mixture, making the reaction suitable for industrial processes 9
- 10. âĒ Generally aryl halides react sluggishly. âĒ By-products such as self-coupling products are formed because of solvent-dissolved oxygen. DISADVANTAGES 10
- 11. âĒ Development of catalysts facilitating coupling of unreactive aryl halides. Eg: Ni catalysts have been used as catalysts for coupling aryl chlorides, for the following reasons. ï No handling of air or moisture - sensitive material is necessary. ï A wide variety of functional groups are tolerated and unsymmetrical biaryls are obtained in high yields. ADVANCEMENTS 11
- 12. âĒ Xenbucin 1, an analgesic drug, was synthesized in 4 steps using two different routes. The biaryl fragment could successfully be produced via a Pd/C catalysed Suzuki coupling [5] 12 SYNTHETIC APPLICATIONS
- 13. âĒ Boscalid, a fungicide is prepared via Suzuki reaction.[6] 13
- 14. âĒ In Valsartan, the biaryls can easily be prepared by this reaction. [6] 14
- 15. âĒ The formal total synthesis of Oximidine by G.A.Molander et al. was done by using this reaction. [4] 15
- 16. âĒ The key step in the total synthesis of Myxalamide A by C.H. Heathcock et al. was Suzuki Cross-Coupling. 16
- 17. âĒ The natural anti tumour product Epothilone A was synthesized in the laboratory by J.S. Panek by using the Suzuki Cross Coupling Reaction. 17
- 18. REFERENCE 1. Jie Jack Li; Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications Fifth Edition; Springer Science & Business Media, 30-Jan-2014; Page no. 544 2. Benny K G Theng; Clay Mineral Catalysis of Organic Reactions; CRC Press, 27-Jul-2018; Page No.2016 3. Ranjit S. Dhillon; Hydroboration and Organic Synthesis : 9-Borabicclo [3.3.1] nonane (9-BBN); Springer Science & Business Media; 01 - May - 2007; Page No. 523 4. Laszlo Kurti, Barbara Czako; Strategic Applications of Named Reactions in Organic Synthesis; Elsevier, 29-Apr-2005; Page no. 37 18
- 19. 4. Noora KuulojaTuula KylmÃĪlÃĪYoujun XuRobert FranzÃĐn; Synthesis of Xenbucin using Suzuki reaction catalyzed by Pd/C in water; Central European Journal of Chemistry; September 2008, Volume 6, Issue 3, pp 390â392 5. .A. MAUREEN ROUHI, C&EN WASHINGTON;FINE CHEMICALS Suzuki- coupling chemistry takes hold in commercial practice, from small-scale synthesis of screening compounds to industrial production of active ingredients; American Chemical Society; September 6, 2004 Volume 82, Number 36 pp. 49-58 19
- 20. THANK YOU 20

![Suzuki reaction is the Pd catalysed cross coupling reaction[1]
between the boronic / organoboronic acids / organoboranes with
organic halides[2], triflates, etc under basic conditions[3] which leads
to the formation of carbon carbon single bonds.[4]
ïThis reaction is commonly used for synthesising biaryl
compounds.
2](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-2-320.jpg)







![âĒ Xenbucin 1, an analgesic drug, was synthesized in 4 steps using two
different routes. The biaryl fragment could successfully be produced
via a Pd/C catalysed Suzuki coupling [5]
12
SYNTHETIC APPLICATIONS](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-12-320.jpg)
![âĒ Boscalid, a fungicide is prepared via Suzuki reaction.[6]
13](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-13-320.jpg)
![âĒ In Valsartan, the biaryls can easily be prepared by this reaction. [6]
14](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-14-320.jpg)
![âĒ The formal total synthesis of Oximidine by G.A.Molander et al. was
done by using this reaction. [4]
15](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-15-320.jpg)


![REFERENCE
1. Jie Jack Li; Name Reactions: A Collection of Detailed Mechanisms and
Synthetic Applications Fifth Edition; Springer Science & Business Media,
30-Jan-2014; Page no. 544
2. Benny K G Theng; Clay Mineral Catalysis of Organic Reactions; CRC
Press, 27-Jul-2018; Page No.2016
3. Ranjit S. Dhillon; Hydroboration and Organic Synthesis : 9-Borabicclo
[3.3.1] nonane (9-BBN); Springer Science & Business Media; 01 - May -
2007; Page No. 523
4. Laszlo Kurti, Barbara Czako; Strategic Applications of Named Reactions
in Organic Synthesis; Elsevier, 29-Apr-2005; Page no. 37
18](https://image.slidesharecdn.com/suzukireaction-190218212358/85/Suzuki-reaction-18-320.jpg)

