Synthetic Strategies For 14 C Labelling Of Drug Molecules
- 1. Synthetic Strategies for 14 C Labelling of Drug Molecules Dr Sean L Kitson [email_address]
- 2. Objective This lecture will focus on a brief introduction to ‘what is’ carbon-14 Then leading onto some synthetic strategies towards labelling potential drug molecules with carbon-14
- 3. Agenda Introduction to 14 C 14 C Synthetic Strategies: [ 14 C]XEN-D0401 [ 14 C]Apomorphine [ 14 C]Combretastin-A1 [5- 14 C]Hex-5-ynoic acid [ 14 C]ZT-1 Conclusion
- 4. Introduction to 14 C
- 5. Discovery of 14 C Martin Kamen & Sam Ruben (27-FEB-1940) T 1/2 ~ 5730 Years
- 6. 14 C Starting Materials
- 7. Barium 14 C carbonate staircase
- 8. 14 C Radiotracer In pharmaceutical research 14 C is used as a tracer to ensure that potential drugs are metabolized without forming harmful by-products – ADMET Studies The 14 C label should ideally form part of the compounds molecular skeleton
- 9. 14 C Synthetic Strategies 14 C labelling
- 10. Target: [ 14 C]XEN-D0401 XEN-D0401 is a novel and selective small molecule activator of the large-conductance calcium-activated potassium channel (known as the BK channel) Currently in Phase 1 development for the treatment of overactive bladder (OAB ) * = 14 C Label
- 11. 14 C Starting Material
- 12. Generation of ‘Dry’ 14 CO 2
- 13. Carboxylation
- 14. O -Methylation
- 15. Ìý
- 16. S L Kitson, S J Jones et al . J Label Compd. Radiopharm 2010 , 53, 140-146
- 17. Key Step: A one pot construction of the quinolin-2-one ring under strong base
- 18. Aporphine skeleton ‘naturally occurring’ S L Kitson. J Label Compd Radiopharm; 2007 , 50, 290-294 S L Kitson. J Label Compd Radiopharm; 2006 , 49, 517-531
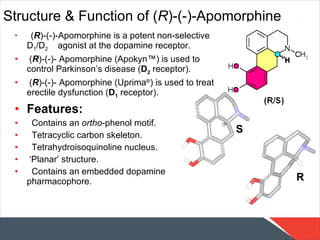
- 19. Structure & Function of ( R )-(-)-Apomorphine ( R )-(-)-Apomorphine is a potent non-selective D 1 /D 2 agonist at the dopamine receptor. ( R )-(-)- Apomorphine (Apokyn ™ ) is used to control Parkinson’s disease ( D 2 receptor). ( R )-(-)- Apomorphine (Uprima ® ) is used to treat erectile dysfunction ( D 1 receptor). Features: Contains an ortho -phenol motif. Tetracyclic carbon skeleton. Tetrahydroisoquinoline nucleus. ‘ Planar’ structure. Contains an embedded dopamine pharmacophore.
- 20. R etrosynthesis of Apomorphine
- 21. Synthesis of N- [carboxyl- 14 C]acetamide
- 23. N -Methylation Reduction Step
- 26. ( R )-(-)-[6a- 14 C]Apomorphine PLRP-S column (CH 3 CN:HCl aq) Specific Activity: 55mCi/mmol Radiochemical purity => 98% Radiochiral purity => 99%
- 27. Combretastatin A-1 Combretastatin A-1 is a natural product first isolated from the African bush willow tree in the 1980s Chemotherapeutic properties by acting on the tumour vasculature, leading to blood flow shut down and ultimately tumour death
- 28. Target: [methyl- 14 C]Combretastatin A-1 diphosphate Treatment of solid tumours The pro-drug undergoes in vivo de-phosphorylation to generate the active agent combretastatin A-1
- 29. 14 C Synthetic route R T Brown et al . J Label Compd. Radiopharm 2009 , 52, 567-570
- 30. Target: [5- 14 C]Hex-5-ynoic acid [ 14 C]Acetylenes
- 31. [ 14 C]Acetylenes: Ohira Bestmann Reagent
- 32. 14 C Acetylene Synthesis Overall Radiochemical Yield 29 % from [ 14 C]KCN
- 33. [ 14 C]ZT-1: a new generation of acetylcholinesterase (AChE) inhibitors L Leman, S L Kitson et al. J. Label Compd. Radiopharm 2011 , 720-730
- 34. 14 C Synthesis * = U- 14 C
- 35. [ 14 C]-ZT-1
- 36. Conclusion When designing a 14 C labelled synthesis it is important to consider the following: Identify simple starting materials from the barium 14 C carbonate ‘staircase’ which are commercially available or alternatively easily made Plan , develop and execute the synthetic methodology to the final drug substance. This approach can often restrict the position of the label in the drug and will cause a change in the drug purity profile from the original laboratory synthesis route Locate a biologically stable position for the 14 C label
- 37. 14 C Radiochemistry Laboratory
![Synthetic Strategies for 14 C Labelling of Drug Molecules Dr Sean L Kitson [email_address]](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-1-320.jpg)

![Agenda Introduction to 14 C 14 C Synthetic Strategies: [ 14 C]XEN-D0401 [ 14 C]Apomorphine [ 14 C]Combretastin-A1 [5- 14 C]Hex-5-ynoic acid [ 14 C]ZT-1 Conclusion](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-3-320.jpg)






![Target: [ 14 C]XEN-D0401 XEN-D0401 is a novel and selective small molecule activator of the large-conductance calcium-activated potassium channel (known as the BK channel) Currently in Phase 1 development for the treatment of overactive bladder (OAB ) * = 14 C Label](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-10-320.jpg)









![Synthesis of N- [carboxyl- 14 C]acetamide](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-21-320.jpg)




![( R )-(-)-[6a- 14 C]Apomorphine PLRP-S column (CH 3 CN:HCl aq) Specific Activity: 55mCi/mmol Radiochemical purity => 98% Radiochiral purity => 99%](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-26-320.jpg)

![Target: [methyl- 14 C]Combretastatin A-1 diphosphate Treatment of solid tumours The pro-drug undergoes in vivo de-phosphorylation to generate the active agent combretastatin A-1](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-28-320.jpg)

![Target: [5- 14 C]Hex-5-ynoic acid [ 14 C]Acetylenes](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-30-320.jpg)
![[ 14 C]Acetylenes: Ohira Bestmann Reagent](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-31-320.jpg)
![14 C Acetylene Synthesis Overall Radiochemical Yield 29 % from [ 14 C]KCN](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-32-320.jpg)
![[ 14 C]ZT-1: a new generation of acetylcholinesterase (AChE) inhibitors L Leman, S L Kitson et al. J. Label Compd. Radiopharm 2011 , 720-730](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-33-320.jpg)

![[ 14 C]-ZT-1](https://image.slidesharecdn.com/syntheticstrategiesfor14clabellingofdrugmolecules-13258511082832-phpapp02-120106055935-phpapp02/85/Synthetic-Strategies-For-14-C-Labelling-Of-Drug-Molecules-35-320.jpg)

