T12 IB Chemistry Atomic structure
- 1. 12.1- Electrons in atoms
- 2. Lesson outlineŌĆ” ŌĆó Lesson 1- Topic 2 review. ŌĆó Lesson 2- What does ionization energy (I.E.) tell us about the structure of an atom? ŌĆó Lesson 3- Topic 2/12 review questions.
- 3. Lesson 1- Topic 2 review . Level 4: Recall that an isotope is a variant of an element due to more or less neutrons. Level 7: Explain how emission & absorption spectra were used to come up with the idea of atomic orbitals (s,p,d etc.) Level 5/6: determine the % abundance of an isotope from given data.
- 4. Topic 2 review ŌĆó See worksheet and answers. ŌĆó You can also try this quizlet: https://quizlet.com/167940560/ib-chemistry- atomic-structure-flash-cards/
- 5. Lesson 2- What does ionization energy (I.E.) tell us about the structure of an atom? Level 4: State what the definition is for first ionization energy. Level 7: Explain how trends in successive I.E. show evidence for energy levels and sub levels. Level 5/6: Justify the general trend in first I.E. for periods 1, 2 & 3.
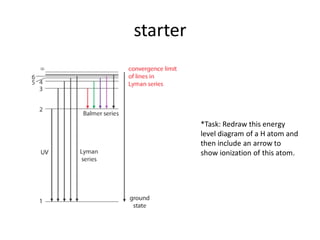
- 6. starter *Task: Redraw this energy level diagram of a H atom and then include an arrow to show ionization of this atom.
- 7. Ionization energy (I.E.) The first ionization energy is the energy needed to remove one mole of electrons from the ground state of one mole of the gaseous atom. In short: H(g)’āĀ H+ (g) + e- KJ.mol-1 https://www.youtube.com/watch?v=0EnK4EXLDUc (short video clip 1.20min)
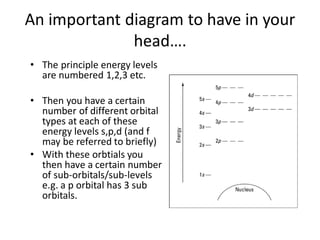
- 8. An important diagram to have in your headŌĆ”. ŌĆó The principle energy levels are numbered 1,2,3 etc. ŌĆó Then you have a certain number of different orbital types at each of these energy levels s,p,d (and f may be referred to briefly) ŌĆó With these orbtials you then have a certain number of sub-orbitals/sub-levels e.g. a p orbital has 3 sub orbitals.
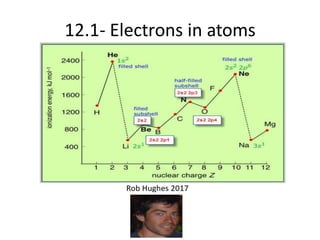
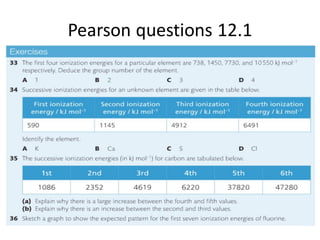
- 9. Successive I.E. & ŌĆśprincipleŌĆÖ energy levels. (e.g. Aluminium) *8min to justify using e configs. Q. Why is there a jump between the 3rd & 4th and the 11th&12th I.E.? Q. Why is there a need for a log scale on the y axis?
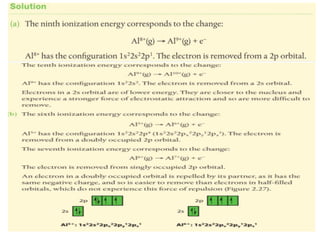
- 10. Successive I.E. & sub- levels. (e.g. Aluminium) (a) Explain, using e-configs, why there is a large increase between the ninth and tenth I.E. (b) Explain why the increase between the sixth and seventh values is greater than the increase between the fifth and sixth values.
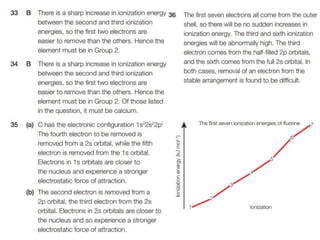
- 12. Trends in first I.E. across a periodŌĆ” 1. What is the general trend in I.E. across a period/row? 2. What is the general trend in I.E. down the periodic table? 3. What is the reason for the anomaly/discontinuity between Be and B and between N and O? (which is also repeated in row 3)
- 15. Topic 2/12 review See practice questionsŌĆ”.. (from inthinking & Pearson)