Tabex 2
- 1. Is Cytisine at least as effective as NRT for smoking cessation?
- 2. â BACKGROUND â Cytisine is a plant-based Alkaloid found in members of the Leguminosae family,a partial agonist that binds the nicotinic acetylcholine receptors (nAChRs) â Cytisine is used for smoking cessation,it has been available both with and without prescription for smoking cessation since 1960s largely in Eastern Europe â Cytisine is a generic agent currently manufactured by Sopharma as Tabex and Aflofarm Pharma as Desmoxan
- 3. â 4 Systematic reviews report Cytisine to be superior to placebo for short-term and long-term abstinence and almost doubles the chances of quiting at 6 months â Given that no trials have compared Cytisine with Nicotine Replacement Therapy(NRT),we designed a non-inferiority trial to investigate wether Cytisine was at least as effective as NRT
- 4. â METHODS â 1310 -(655 per group) adult smokers were randomly assigned in a1:1 ratio to receive Cytisine for 25 days or NRT for 8 weeks â Participants-Inclusion criteria â Smokers motivated to qiut(were recruited through the New Zealand Quitline â To be at lesast 18 of age â Dayli smokers â Able to provide verbal consent â Have access to telephone â Participants-Exclusion criteria â Pregnant/Breastfeeding â Non-dayli smokers â Current users of NRT products â Currently enrolled in another smoking cessation study â Heart attack,uncontrolled high blood pressure, schizophrenia
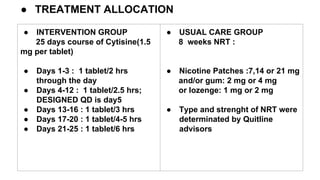
- 5. â TREATMENT ALLOCATION â INTERVENTION GROUP 25 days course of Cytisine(1.5 mg per tablet) â Days 1-3 : 1 tablet/2 hrs through the day â Days 4-12 : 1 tablet/2.5 hrs; DESIGNED QD is day5 â Days 13-16 : 1 tablet/3 hrs â Days 17-20 : 1 tablet/4-5 hrs â Days 21-25 : 1 tablet/6 hrs â USUAL CARE GROUP 8 weeks NRT : â Nicotine Patches :7,14 or 21 mg and/or gum: 2 mg or 4 mg or lozenge: 1 mg or 2 mg â Type and strenght of NRT were determinated by Quitline advisors
- 6. â TREATMENT REGIME â CYTISINE/TABEX Days 1-3 : 1.5 mg/2 hours Days 4-12 :1.5 mg /2.5 hours DESIGNED QIUT DAY- DAY 5 Days 13-16 : 1.5 mg/3 hours Days 17-20 : 1.5 mg/4-5 hours Days 21 -25 : 1.5 mg/6 hours â VARENICLINE/CHAMPIX Days 1-3 : 0.5 mg once daily Days 4-7 : 0.5 mg twice daily DESIGNED QUIT DAY- DAY 7-14 Day 8 to week 12 :n1n mg twice daily
- 7. â BASELINE CHARACTERISTIC OF THE PARTICIPANTS CHARACTERISTICS CYTISINE N=655 NRT N=655 FEMALE SEX-no(%) 372(57%) 372(57%) AGE(yrs) 37.8 38.4 CIGARETTES SMOKED PER DAY 19.3 19.0 CIGARETTE DEPENDENCE 5.4 5.3 AGE STARTED SMOKING(yrs) 15.2 15.5
- 8. â OUTCOMES â Primary outcomes â continuous abstinence since quit day â Secondary outcomes - assessed at 1 week and 1,2, and 6 months(after quit day) â total number of Cytisine tablets taken or the type,strenght and amount of NRT â 7-Day piont preavalance for abstinence(no cigarettes,not a single puff in the previous 7 days)