Term 1 Grade 8 ATOMS.pptx
- 1. TERM 1 GRADE 8
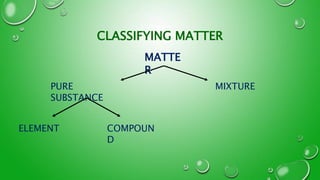
- 2. LETS START FROM THE BEGINNINGŌĆ”ŌĆ”. WHAT IS MATTER? MATTER IS EVERYTHING THAT HAS A MASS AND OCCUPIES SPACE MATTER ELEMENTS ATOMS
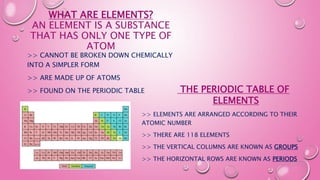
- 3. WHAT ARE ELEMENTS? AN ELEMENT IS A SUBSTANCE THAT HAS ONLY ONE TYPE OF ATOM >> CANNOT BE BROKEN DOWN CHEMICALLY INTO A SIMPLER FORM >> ARE MADE UP OF ATOMS >> FOUND ON THE PERIODIC TABLE THE PERIODIC TABLE OF ELEMENTS >> ELEMENTS ARE ARRANGED ACCORDING TO THEIR ATOMIC NUMBER >> THERE ARE 118 ELEMENTS >> THE VERTICAL COLUMNS ARE KNOWN AS GROUPS >> THE HORIZONTAL ROWS ARE KNOWN AS PERIODS
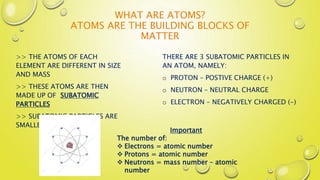
- 4. WHAT ARE ATOMS? ATOMS ARE THE BUILDING BLOCKS OF MATTER >> THE ATOMS OF EACH ELEMENT ARE DIFFERENT IN SIZE AND MASS >> THESE ATOMS ARE THEN MADE UP OF SUBATOMIC PARTICLES >> SUBATOMIC PARTICLES ARE SMALLER THAN AN ATOM THERE ARE 3 SUBATOMIC PARTICLES IN AN ATOM, NAMELY: o PROTON ŌĆō POSTIVE CHARGE (+) o NEUTRON ŌĆō NEUTRAL CHARGE o ELECTRON ŌĆō NEGATIVELY CHARGED (-) Important The number of: ’üČ Electrons = atomic number ’üČ Protons = atomic number ’üČ Neutrons = mass number ŌĆō atomic number
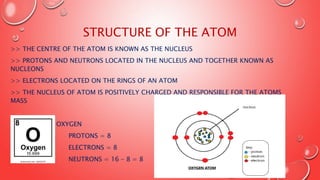
- 5. STRUCTURE OF THE ATOM >> THE CENTRE OF THE ATOM IS KNOWN AS THE NUCLEUS >> PROTONS AND NEUTRONS LOCATED IN THE NUCLEUS AND TOGETHER KNOWN AS NUCLEONS >> ELECTRONS LOCATED ON THE RINGS OF AN ATOM >> THE NUCLEUS OF ATOM IS POSITIVELY CHARGED AND RESPONSIBLE FOR THE ATOMS MASS FOR EXAMPLE: OXYGEN PROTONS = 8 ELECTRONS = 8 NEUTRONS = 16 ŌĆō 8 = 8
- 6. PRACTICE DRAW AN ATOM FOR THE FOLLOWING ELEMENTS : 1. CARBON 2. NITROGEN 3. FLUORINE 4. LITHIUM
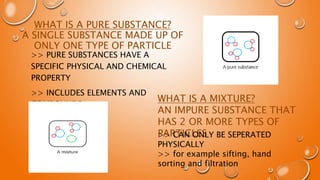
- 8. WHAT IS A PURE SUBSTANCE? A SINGLE SUBSTANCE MADE UP OF ONLY ONE TYPE OF PARTICLE >> PURE SUBSTANCES HAVE A SPECIFIC PHYSICAL AND CHEMICAL PROPERTY >> INCLUDES ELEMENTS AND COMPOUNDS WHAT IS A MIXTURE? AN IMPURE SUBSTANCE THAT HAS 2 OR MORE TYPES OF PARTICLES >> CAN ONLY BE SEPERATED PHYSICALLY >> for example sifting, hand sorting and filtration
- 9. WHAT ARE COMPOUNDS? ARE SUBSTANCES MADE UP OF 2 OR MORE ELEMENTS >> COMPOUNDS ARE CHEMICALLY BONDED >> CANNOT BE SEPERATED PHYSICALLY