testing method of WWTP parameters using labs
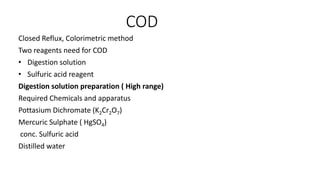
- 1. COD Closed Reflux, Colorimetric method Two reagents need for COD ŌĆó Digestion solution ŌĆó Sulfuric acid reagent Digestion solution preparation ( High range) Required Chemicals and apparatus Pottasium Dichromate (K2Cr2O7) Mercuric Sulphate ( HgSO4) conc. Sulfuric acid Distilled water
- 2. Apparatus ŌĆó 1000 ml Volumetric Flask ŌĆó Glass rod ŌĆó Funnel About 10.216g K2Cr2O7 ( Primary standard grade, previously dried at 150 ┬░C for 2h) was added in to the 1000 ml volumetric flask. Then 500 ml distilled water was added in to it and mixed well. After that 167 ml conc. Sulfuric acid was added gently in to it. About 33.3 g HgSO4 was added and dissolved. Solution was cooled to room temperature and dilute to 1000 ml by using distilled water.
- 3. Sulfuric acid Reagent preparation Required Chemicals and apparatus ŌĆó Silver Sulfate ( Ag2SO4) ŌĆó Sulfuric acid Apparatus ŌĆó 500 ml beaker ŌĆó Glass rod
- 4. ŌĆó Add Ag2SO4 powder, to Conc. Sulfuric acid at the rate of 5.5g Ag2SO4/Kg H2SO4. Let stand 1 to 2 d to dissolve. ŌĆó Conversion of H2SO4 mass to volume Density= ØæÜØæÄØæĀØæĀ ØæēØæ£ØæÖØæóØæÜØæÆ 1.83 gcm-3 = 1000 Øæö ØæēØæ£ØæÖØæóØæÜØæÆ = 546.44 ml 5.5 g Ag2SO4 was added in to the 500 ml beaker and 546.44 ml H2SO4 was added in to it. Let stand 1 to 2 days to dissolve.
- 5. Vial Preparation ŌĆó 2.5 ml waste water sample was added in to the COD vial. 1.5 ml digestion solution was added in to it. After that 3.5 ml sulfuric acid reagent was added in to the vial and mixed well. Blank preparation 2.5 ml distilled water was added in to the COD vial. 1.5 ml digestion solution was added in to it. After that 3.5 ml sulfuric acid reagent was added in to the vial and mixed well.
- 6. Digest the sample ŌĆó COD digester machine was preheated at 150 ┬░C. After that vials were placed in to the machine and maintained 150 ┬░C for 2h. Finally vials were cooled to room temperature and read the value using colorimeter. ŌĆó Reference: