The Collision Theory
- 2. The collision theory states that gas-phase chemical reaction occurs when molecules colliding have sufficient kinetic energy.
- 3. What is activation energy? Activation energy is the energy required to proceed and reach the transition state.
- 4. Reaction rate Reaction rate is intuitively defines as how quickly or slowly a reaction takes place.
- 5. Factors affecting reaction rate ď‚—Temperature ď‚—Surface area ď‚—Concentration of reactants ď‚—Catalyst
- 6. Temperature If the temperature is increased: * the reactant particles move more quickly * the reactant particles have more kinetic energy * the reaction rate increases
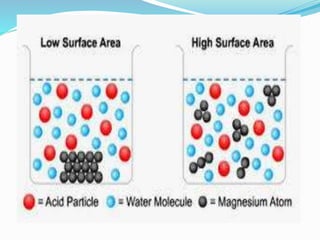
- 8. Surface area Larger surface area would have a larger space of collision between particles of a reaction.
- 10. Concentration of reactants If the concentration is increased: * the reactant particles become more crowded * there is a greater chance of the particles colliding; * the rate of reaction increases
- 12. Catalyst Catalyst is a substance that makes a chemical reaction faster.